Abstract
Purpose. Control of the transport of molecules into the nucleus represents a key regulatory mechanism for differentiation, transformation, and signal transduction. Permeabilization of the nuclear envelope by physical methods can have applications in gene therapy. Laser-induced pressure transients can produce temporary aqueous pores analogous to those produced by electroporation and that the cells can survive this procedure. In this study, we examine the role of the pressure transients in creating similar pores in the nuclear envelope.
Methods. The target human peripheral blood mononuclear cells in a 62 μM 72 kDa fluoresceinated dextran solution were exposed to the pressure transients generated by laser ablation. An in vitro fluorescence confocal microscope was used to visualize and quantify the fluoresceinated dextran in the cytoplasmic and nuclear compartments.
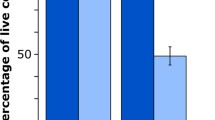
Results. In contrast to electroporation, the pressure transients could deliver 72 kDa fluoresceinated dextrans, which are normally excluded by the nucleus, across the nuclear envelope into the nucleus. In addition to creating pores in the plasma membrane, temporary pores were also created in the nuclear envelope following exposure to pressure transients.
Conclusion. The production of temporary nuclear pores could provide a unique resource for drug-delivery and gene therapy.
Similar content being viewed by others
References
R. Peters. Nuclear envelope permeability measured by fluorescence microphotolysis of single liver cell nuclei. J. Biol. Chem. 258:11427–11429 (1983).
R. Peters, I. Lang, M. Scholz, B. Schulz, and F. Kayne. Fluorescence microphotolysis to measure nucleocytoplasmic transport in vivo and in vitro. Biochem. Soc. Trans. 14:821–822 (1986).
I. Lang, M. Scholz, and R. Peters. Molecular mobility of nucleocytoplasmic flux in hepatoma cells. J. Cell Biol. 102:1183–1190 (1986).
R. Peters. Nucleo-cytoplasmic flux and intracellular mobility in single hepatocytes measured by fluorescence microphotolysis. EMBO J. 3:1831–1836 (1984).
B. Schulz and R. Peters. Nucleocytoplasmic protein traffic in single mammalian cells studied by fluorescence microphotolysis. Biochim. Biophys. Acta 930:419–431 (1987).
M. Scholz, C. Gross-Johannböcke, and R. Peters. Measurement of nucleo-cytoplasmic transport by fluorescence microphotolysis and laser scanning microscopy. Cell Biol. Int. Rep. 12:709–727 (1988).
S. Lee, T. Anderson, H. Zhang, T. J. Flotte, and A. G. Doukas. Alteration of cell membrane by stress waves in vitro. Ultrasound Med. Biol. 22:1285–1293 (1996).
L. M. Lyamshev. Optoacoustic sources of sound. Sov. Phys. Usp. 24:977–995 (1981).
S. E. Mulholland, S. Lee, D. J. McAuliffe, and A. G. Doukas. Cell loading with laser-generated stress waves: the role of the stress gradient. Pharm. Res. 16:514–518 (1999).
Y. Yashima, D. J. McAuliffe, S. L. Jacques, and T. J. Flotte. Laser-induced photoacoustic injury of skin: effect of inertial confinement. Lasers Surg. Med. 11:62–68 (1991).
A. G. Doukas, D. J. McAuliffe, and T. J. Flotte. Biological effects of laser-induced shock waves: structural and functional cell damage in vitro. Ultrasound Med. Biol. 19:137–146 (1993).
S. Lee, D. J. McAuliffe, T. J. Flotte, N. Kollias, and A. G. Doukas. Photomechanical transdermal delivery: The effect of laser confinement. Lasers Surg. Med. 28:344–347 (2001).
C. R. Phipps Jr., T. P. Turner, R. F. Harrison, G. W. York, W. Z. Osborne, G. K. Anderson, X. F. Corlis, L. C. Haynes, H. S. Steele, K. C. Spicochi, and T. R. King. Impulse coupling targets in vacuum by KrF, HF, and CO2 single pulse laser. J. Appl. Phys. 64:1083–1096 (1988).
S. A. Glantz. Primer of Biostatistics, 3rd edition, McGraw-Hill, New York, 1992.
J. Liu, T. N. Lewis, and M. R. Prausnitz. Non-invasive assessment and control of ultrasound-mediated membrane permeabilization. Pharm. Res. 15:918–924 (1998).
M. Delius and G. Adams. Shock wave permealization with ribosome inactivating proteins: a new approach to tumor therapy. Cancer Res. 59:5227–5232 (1999).
M. Delius, F. Ueberle, and S. Gambihler. Acoustic energy determines haemoglobin release from erythrocytes by extracorporeal shock waves in vitro. Ultrasound Med. Biol. 21:707–710 (1995).
S. Gambihler, M. Delius, and J. W. Ellwart. Permeabilization of the plasma-membrane of L1210 mouse leukemia cells using lithotripter shock-waves. J. Membr. Biol. 141:267–275 (1994).
U. Lauer, E. Burgelt, Z. Squire, K. Messmer, P. H. Hofschneider, M. Gregor, and M. Delius. Shock wave permeabilization as a new gene transfer method. Gene Ther. 4:710–715 (1997).
Y. Yashima, D. J. McAuliffe, and T. J. Flotte. Cell selectivity to laser-induced photoacoustic injury of skin. Lasers Surg. Med. 10:280–283 (1990).
B. J. Wong, M. R. Dickinson, and M. W. Berns. and J. Neev. Identification of photoacoustic transients during pulsed laser ablation of the human temporal bone: an experimental model. J. Clin. Laser Med. Surg. 14:385–392 (1996).
S. Lee, D. J. McAuliffe, H. Zhang, Z. Xu, J. Taitelbaum, T. J. Flotte, and A. G. Doukas. Stress-wave-induced membrane permeation of red blood cells is facilitated by aquaporins. Ultrasound Med. Biol. 23:1089–1094 (1997).
J. S. Soughayer, T. Krasieva, S. C. Jacobson, J. M. Ramsey, B. J. Tromberg, and N. L. Allbritton. Characterization of cellular optoporation with distance. Anal. Chem. 72:1342–1347 (2000).
M. Bier, S. M. Hammer, D. J. Canaday, and R. C. Lee. Kinetics of sealing for transient electropores in isolated mammalian skeletal muscle cells. Bioelectromagnetics 20:194–201 (1999).
D. C. Chang and T. S. Reese. Changes in membrane structure induced by electroporation as revealed by rapid-freezing electron microscopy. Biophys. J. 58:1–12 (1990).
S. Y. Ho and G. S. Mittal. Electroporation of cell membranes: a review. Crit. Rev. Biotechnol. 16:349–362 (1996).
A. Coonrod, F. Q. Li, and M. Horwitz. On the mechanism of DNA transfection: efficient gene transfer without viruses. Gene Ther. 4:1313–1321 (1997).
D. C. Lamb, J. Tribble, A. G. Doukas, T. J. Flotte, R. H. Ossoff, and L. Reinisch. Custom designed acoustic pulses. J. Biomed. Optics 4:217–223 (1999).
M. Delius, F. Ueberle, and W. Eisenmenger. Extracorporeal shock waves act by shock wave-gas bubble interaction. Ultrasound Med. Biol. 24:1055–1059 (1998).
R. C. Mulligan. The basic science of gene therapy. Science 260:926–932 (1993).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lin, TY.D., McAuliffe, D.J., Michaud, N. et al. Nuclear Transport by Laser-Induced Pressure Transients. Pharm Res 20, 879–883 (2003). https://doi.org/10.1023/A:1023835219041
Issue Date:
DOI: https://doi.org/10.1023/A:1023835219041