Abstract
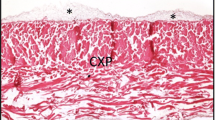
To study the spatiotemporal processes of angiogenesis during a foreign body reaction (FBR), biodegradable bovine collagen type-1 (COL-I) discs were implanted in mice for a period up to 28 days. The cellular infiltration (consisting mainly of macrophages, giant cells and fibroblasts), and the extent of neovascularization into the discs were determined. Also the expression levels and/or distribution of the endothelial cell markers von Willebrand factor (vWF), platelet endothelial cell adhesion molecule-1 (PECAM-1)/CD31, MECA-32 antigens and endomucin, and of the basal lamina marker collagen type IV (Coll IV) were analysed. In time, a strong neovascularization of the discs was observed, with frequently occurring vascular sprouting, and intussusceptive growth of vessels. In this model, vWF, MECA-32 and endomucin antibodies often failed to stain neovessels in the COL-I discs. In contrast, staining for collagen IV basal lamina component in combination with CD31 covered the complete range of neo-vessels. We conclude that the model described in this study is a useful model to study FBR induced angiogenesis because of the active neovascularization taking place during prolonged periods of time.
Similar content being viewed by others
References
Patan S, Haenni B, Burri PH. Implementation of intussusceptive microvascular growth in the chicken chorioallantoic membrane (CAM): 1. Pillar formation byfolding of the capillarywall. Microvasc Res 1996; 51: 80–98.
Colville-Nash PR, Scott DL. Angiogenesis and rheumatoid arthritis: Pathogenic and therapeutic implications. Ann Rheum Dis 1992; 51: 919–25.
Ross R. Atherosclerosis is an inflammatorydisease. Am Heart J 1999; 138: S419–20.
Griffioen AW, Molema G. Angiogenesis: Potentials for pharmacologic intervention in the treatment of cancer, cardiovascular diseases, and chronic inflammation. Pharmacol Rev 2000; 52: 237–68.
Coleman DL, King RN, Andrade JD. The foreign bodyreaction: A chronic inflammatoryresponse. J Biomed Mater Res 1974; 8: 199–211.
Anselme K, Bacques C, Charriere G et al. Tissue reaction to subcutaneous implantation of a collagen sponge. A histological, ultrastructural, and immunological study. J Biomed Mater Res 1990; 24: 689–703.
van Wachem PB, van Luyn MJ, Olde Damink LH et al. Biocompatibilityand tissue regenerating capacity of crosslinked dermal sheep collagen. J Biomed Mater Res 1994; 28: 353–63.
Fournier N, Doillon CJ. Biological molecule-impregnated polyester: An in vivo angiogenesis study. Biomaterials 1996; 17: 1659–65.
Padera RF, Colton CK. Time course of membrane microarchitecture-driven neovascularization. Biomaterials 1996; 17: 277–84.
Pieper JS, van Wachem PB, van Luyn MJA et al. Attachment of glycosaminoglycans to collagenous matrices modulates the tissue response in rats. Biomaterials 2000; 21: 1689–99.
Khouw IM, van Wachem PB, Molema G et al. The foreign body reaction to a biodegradable biomaterial differs between rats and mice. J Biomed Mater Res 2000; 52: 439–46.
Altman DG. Practical Statistics for Medical Research. London: Chapman & Hall 1991.
Streeter PR, Berg EL, Rouse BT et al. A tissue-specific endothelial cell molecule involved in lymphocyte homing. Nature 1988; 331: 41–6.
Morgan SM, Samulowitz U, Darley L et al. Biochemical characterization and molecular cloning of a novel endothelial-specific sialomucin. Blood 1999; 93: 165–75.
Semenza GL. Hypoxia-inducible factor 1: Master regulator of O2 homeostasis. Curr Opin Genet Dev 1998; 8: 588–94.
Sunderkotter C, Steinbrink K, Goebeler M et al. Macrophages and angiogenesis. J Leuk Biol 1994; 55: 410–22.
Jerdan JA, Michels RG, Glaser BM. Extracellular matrix of newly forming vessels-an immunohistochemical study. Microvasc Res 1991; 42: 255–65.
Kuwashima Y, Uehara T, Kurosumi M et al. Basement membrane status in undifferentiated carcinomas of the ovary. Immunohistochemical distribution of type IV collagen and laminin. Eur J Gynaecol Oncol 1995; 16: 181–6.
Ruiter DJ, Schlingemann RO, Westphal JR et al. Angiogenesis in wound healing and tumor metastasis. Behring Inst Mitt 1993; 92: 258–72.
Schlingemann RO, Rietveld FJ, Kwaspen F et al. Differential expression of markers for endothelial cells, pericytes, and basal lamina in the microvasculature of tumors and granulation tissue. Am J Pathol 1991; 138: 1335–47.
Albelda SM, Muller WA, Buck CA, Newman PJ. Molecular and cellular properties of PECAM-1 (endoCAM/CD31): A novel vascular cell-cell adhesion molecule. J Cell Biol 1991; 114: 1059–68.
Romer LH, McLean NV, Yan HC et al. IFN-gamma and TNFalpha induce redistribution of PECAM-1 (CD31) on human endothelial cells. J Immunol 1995; 154: 6582–92.
Stewart RJ, Kashxur TS, Marsden PA. Vascular endothelial platelet endothelial adhesion molecule-1 (PECAM-1) expression is decreased byTNF-alph a and IFN-gamma. Evidence for cytokineinduced destabilization of messenger ribonucleic acid transcripts in bovine endothelial cells. J Immunol 1996; 156: 1221–8.
Rival Y, Del Maschio A, Rabiet MJ et al. Inhibition of platelet endothelial cell adhesion molecule-1 synthesis and leukocyte transmigration in endothelial cells bythe combined action of TNF-alpha and IFN-gamma. J Immunol 1996; 157: 1233–41.
Khouw IMSL, van Wachem PB, de Leij LFMH, van Luyn MJA. Inhibition of the tissue reaction to a biodegradable biomaterial by monoclonal antibodies to IFN-c. J Biomed Mater Res 1998; 41: 202–10.
Cardona MA, Simmons RL, Kaplan SS. TNF and IL-1 generation byhuman monocytes in response to biomaterials. J Biomed Mater Res 1992; 26: 851–9.
DeFife KM, Yun JK, Azeez A et al. Adhesion and cytokine production by monocytes on poly(2-methacryloyloxyethyl phosphorylcholine-co-alkyl methacrylate)-coated polymers. J Biomed Mater Res 1995; 29: 431–9.
Lee AH, Happerfield LC, Bobrow LG, Millis RR. Angiogenesis and inflammation in ductal carcinoma in situ of the breast. J Pathol 1997; 181: 200–6.
Lu J, Kasama T, Kobayashi K et al. Vascular endothelial growth factor expression and regulation of murine collagen-induced arthritis. J Immunol 2000; 164: 5922–7.
Orre M, Rogers PA. Reduced vascular basement-membrane immunostaining in mucinous tumours of the ovary. Int J Cancer 1998; 79: 139–43.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
van Amerongen, M.J., Molema, G., Plantinga, J. et al. Neovascularization and vascular markers in a foreign body reaction to subcutaneously implanted degradable biomaterial in mice. Angiogenesis 5, 173–180 (2002). https://doi.org/10.1023/A:1023822504224
Issue Date:
DOI: https://doi.org/10.1023/A:1023822504224