Abstract
Purpose. It was the purpose of this study to develop a new oral drug delivery system for low molecular weight heparin (LMWH) providing an improved bioavailability and a prolonged therapeutic effect.
Methods. The permeation enhancing polycarbophil-cysteine conjugate (PCP-Cys) used in this study displayed 111.4 ± 6.4 μM thiol groups per gram polymer. Permeation studies on freshly excised intestinal mucosa were performed in Ussing chambers demonstrating a 2-fold improved uptake of heparin as a result of the addition of 0.5% (w/v) PCP-Cys and the permeation mediator glutathione (GSH).
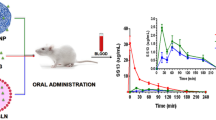
Results. Tablets containing PCP-Cys, GSH, and 279 IU of LMWH showed a sustained drug release over 4 h. To guarantee the swelling of the polymeric carrier matrix in the small intestine tablets were enteric coated. They were orally given to rats. For tablets being based on the thiomer/GSH system an absolute bioavailability of 19.9 ± 9.3% (means ± SD; n = 5) vs. intravenous injection could be achieved, whereas tablets comprising unmodified PCP did not lead to a significant (p < 0.01) heparin concentration in plasma. The permeation enhancing effect and subsequently a therapeutic heparin level was maintained for 24 h after a single dose.
Conclusions. Because of the strong and prolonged lasting permeation enhancing effect of the thiomer/GSH system, the oral bioavailability of LMWH could be significantly improved. This new delivery system represents therefore a promising tool for the oral administration of heparin.
Similar content being viewed by others
References
J. Hirsh, T. E. Warkentin, R. Raschke, C. Granger, E. M. Ohman, and J. E. Dalen. Heparin and low-molecular-weight heparin: mechanisms of action, pharmacokinetics, dosing considerations, monitoring, efficacy, and safety. Chest 114:489–510 (1998).
J. K. Thompson-Ford. Low-molecular-weight-heparin for the treatment of deep vein thrombosis. Pharmacotherapy 18:748–758 (1998).
Y. Lee, S. H. Kim, and Y. Byun. Oral delivery of new heparin derivatives in rats. Pharm. Res. 17:1259–1264 (2000).
Y. Jiao, N. Ubrich, M. Marchand-Arvier, C. Vigneron, M. Hoffman, T. Lecompte, and P. Maincent. In vitro and in vivo evaluation of oral heparin-loaded polymeric nanoparticles in rabbits. Circulation 105:230–235 (2002).
A. Leone-Bay, D. R. Paton, J. Freeman, C. Lercara, D. O'Toole, D. Gschneidner, E. Wang, E. Harris, C. Rosado, T. Rivera, A. DeVincent, M. Tai, F. Mercogliano, R. Agarwal, H. Leipold, and R. A. Baughman. Synthesis and evaluation of compounds that facilitate the gastrointestinal absorption of heparin. J. Med. Chem. 41:1163–1171 (1998).
A. K. Morton, H. E. Edwards, J. C. Allen, and G. O. Phillips. An evaluation of the oral administration of commercial and fractionated heparin samples in rats. Int. J. Pharm. 9:321–335 (1981).
M. Ueno, T. Nakasaki, I. Horikoshi, and N. Sakuragawa. Oral administration of liposomally-entrapped heparin to beagle dogs. Chem. Pharm. Bull. 30:2245–2247 (1982).
B. J. Aungst, H. Saitoh, D. L. Burcham, S. M. Huang, S. A. Mousa, and M. A. Hussain. Enhancement of the intestinal absorption of peptides and non-peptides. J. Control. Release 41:19–31 (1996).
A. Bernkop-Schnürch, V. Schwarz, and S. Steininger. Polymers with thiol groups: a new generation of mucoadhesive polymers? Pharm. Res. 16:876–881 (1999).
A. Bernkop-Schnürch and S. Steininger. Synthesis and characterization of mucoadhesive thiolated polymers. Int. J. Pharm. 194:239–247 (2000).
A. E. Clausen and A. Bernkop-Schnürch. In vitro evaluation of the permeation-enhancing effect of thiolated polycarbophil. J. Pharm. Sci. 89:1253–1261 (2000).
A. E. Clausen and A. Bernkop-Schnürch. Thiolated carboxymethylcellulose: in vitro evaluation of its permeation enhancing effect on peptide drugs. Eur. J. Pharm. Biopharm. 51:25–32 (2001).
C. E. Kast and A. Bernkop-Schnürch. Influence of the molecular mass on the permeation enhancing effect of different poly(acrylates). STP Pharm. 12:351–356 (2002).
M. K. Marschütz, P. Caliceti, and A. Bernkop-Schnürch. Design and in vivo evaluation of an oral delivery system for insulin. Pharm. Res. 17:1468–1474 (2000).
K. Yoshimura, Y. Iwauchi, S. Sugiyama, T. Kuwamura, Y. Odaka, T. Satoh, and H. Kitagawa. Transport of L-cysteine and reduced glutathione through biological membranes. Res. Commun. Chem. Pathol. Pharmacol. 37:171–186 (1982).
A. E. Clausen, C. E. Kast, and A. Bernkop-Schnürch. The role of glutathione in the permeation enhancing effect of thiolated polymers. Pharm. Res. 19:602–608 (2002).
N. Blumenkrantz and G. Asboe-Hansen. New method for quantitative determination of uronic acids. Anal. Chem. 54:484–489 (1973).
S. R. Money and J. W. York. Development of oral heparin therapy for prophylaxis and treatment of deep venous thrombosis. Cardiovasc. Surg. 9:211–218 (2001).
B. J. Aungst. Intestinal permeation enhancers. J. Pharm. Sci. 89:429–442 (2000).
M. D. Gonze, J. D. Manord, A. Leone-Bay, R. A. Baughman, and C. L. Garrard. W. C. III Sternberg, and S. R. Money. Orally administered heparin for preventing deep venous thrombosis. Am. J. Surg. 176:176–178 (1998).
K. Salartash, M. D. Gonze, A. Leone-Bay, and R. A. Baughman. W. C. III Sternberg, and S. R. Money. Oral low-molecular weight heparin and delivery agent prevents jugular venous thrombosis in the rat. J. Vasc. Surg. 30:526–532 (1999).
R. G. Riley, K. L. Green, J. D. Smart, J. Tsibouklis, J. A. Davis, F. Hampson, P. W. Dettmar, and W. R. Wilber. The gastrointestinal transit profile of 14C-labelled poly(acrylic acids): an in vivo study. Biomaterials 22:1861–1867 (2001).
T. Iantomasi, F. Favilli, P. Marraccini, T. Magaldi, P. Bruni, and M. T. Vincenzini. Glutathione transport system in human small intestine epithelial cells. Biochim. Biophys. Acta 1330:274–283 (1997).
T. Rivera, A. Leone-Bay, D. R. Paton, H. Leipold, and R. A. Baughman. Oral delivery of heparin in combination with N-[8-(2-hydroxybenzoyl)amino]caprylate: pharmacological considerations. Pharm. Res. 14:1830–1834 (1997).
R. A. Baughman, S. C. Kapoor, R. Agarwal, J. Kisicki, F. Catella-Lawson, and G. A. FitzGerald. Oral delivery of anticoagulant doses of heparin. A randomized, double blind, controlled study in humans. Circulation 98:1610–1615 (1998).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kast, C.E., Guggi, D., Langoth, N. et al. Development and in Vivo Evaluation of an Oral Delivery System for Low Molecular Weight Heparin Based on Thiolated Polycarbophil. Pharm Res 20, 931–936 (2003). https://doi.org/10.1023/A:1023803706746
Issue Date:
DOI: https://doi.org/10.1023/A:1023803706746