Abstract
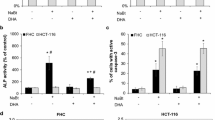
High fecal deoxycholate levels may promote colonic cancer. Phospholipids protect against bile salt-induced cytotoxicity. We therefore aimed to examine whether the dietary phospholipid sphingomyelin could decrease hyperproliferation induced by deoxycholate. In CaCo2 cells, hyperproliferation (by bromodeoxyuridine assay), phosphorylation state of cellular proteins, and apoptosis with concomitant caspase-3 activity were evaluated after incubation with 50–500 μM deoxycholate, with or without sphingomyelin. At 2 and 4 hr of incubation, deoxycholate induced dose-dependent apoptosis, with concomitant caspase-3 activation. At 16 hr, apoptosis had decreased markedly, but there was dose-dependent hyperproliferation (with changed phosphorylation status of cellular proteins) at this time point. Sphingomyelin dose-dependently reduced deoxycholate-induced apoptosis and hyperproliferation. In conclusion, sphingomyelin reduces deoxycholate-induced hyperproliferation and apoptosis. These findings may have implications for colonic cancer prevention by dietary modification.
Similar content being viewed by others
References
Weisburger JH, Wynder EL, Horn CL: Nutritional factors and etiologic mechanisms in the causation of gastrointestinal cancers. Cancer 50:2541-2549, 1982
Bartram HP, Scheppach W, Schmid H, Hofmann A, Dusel G, Richter F, Richter A, Kasper H: Proliferation of human colonic mucosa as an intermediate biomarker of carcinogenesis: effects of butyrate, deoxycholate, calcium, ammonia, and pH. Cancer Res. 53:3283-3288, 1993
Biasco G, Paganelli GM, Owen RW Hill MJ: Faecal bile acids and colorectal cell proliferation. The ECP Colon Cancer Working Group. Eur J Cancer Prev (suppl 2):63-68, 1991
Reddy BS, Watanabe K, Weisburger, JH, Wynder EL: Promoting effect of bile acids in colon carcinogenesis in germ-free and conventional F344 rats. Cancer Res 37:3238-3242, 1977
Marchetti MC, Migliorati G, Moraca R, Riccardi C, Nicoletti I, Fabiani R, Mastrandrea V, Morozzi G: Possible mechanisms involved in apoptosis of colon tumor cell lines induced by deoxycholic acid, short-chain fatty acids, and their mixtures. Nutr Cancer 28:74-80, 1997
Martinez JD, Stratagoules ED, LaRue JM, Powell AA, Gause PR, Craven MT, Payne CM, Powell MB, Gerner EW, Earnest DL: Different bile acids exhibit distinct biological effects: the tumor promoter deoxycholic acid induces apoptosis and the chemopreventive agent ursodeoxycholic acid inhibits cell proliferation. Nutr Cancer 31:111-118, 1998
Velardi ALM, Groen AK, Oude Elferink RP, van der Meer R, Palasciano G, Tytgat GN: Cell type-dependent effect of phospholipid and cholesterol on bile salt cytotoxicity. Gastroenterology 101:457-464, 1991
Moschetta A, vanBerge-Henegouwen GP, Portincasa P, Palasciano G, Groen AK, van Erpecum KJ: Sphingomyelin exhibits greatly enhanced protection compared with egg yolk phosphatidylcholine against detergent bile salts. J Lipid Res 41:916-924, 2000
van Ooteghem NAM, Moschetta A, Rehfeld JF, Samsom M, van Erpecum KJ, vanBerge-Henegouwen GP: Intraduodenal bile salts exert negative feedback control on gallbladder emptying in the fasting state without affecting cholecystokinin release or intestinal motility. Gut 50:669-674, 2002
Duan RD, Nilsson A: Purification of a newly identified alkaline sphingomyelinese in human bile and effects of bile salts and phosphatidylcholine on enzyme activity. Hepatology 26:823-830, 1997
Duan RD, Hertervig E, Nyberg L, Hauge T, Sternby B, Lillienau J, Farooqi A, Nilsson A: Distribution of alkaline sphingomyelinase activity in human beings and animals. Time and species differences. Dig Dis Sci 41:1801-1806, 1996
Nyberg L, Nilsson A, Lundgren P, Duan RD: Localization and capacity of sphingomyelin digestion in the rat intestinal tract. Nutr Biochem 8:112-118, 1997
Nilsson A: Metabolism of sphingomyelin in the intestinal tract of the rat. Biochim Biophys Acta 164:575-584, 1968
Nyberg L, Duan R, Nilsson A: A mutual inhibitory effect on absorption of sphingomyelin and cholesterol. J Nutr Biochem 11:244-249, 2000
Schmelz EM, Dillehay DL, Webb SK, Reiter A, Adams J, Merrill AH Jr: Sphingomyelin consumption suppresses aberrant colonic crypt foci and increases the proportion of adenomas versus adenocarcinomas in CF1 mice treated with 1,2-dimethylhydrazine: implications for dietary sphingolipids and colon carcinogenesis. Cancer Res 56:4936-4941, 1996
Hakomori S: Chemistry of glycosphingolipids. In Handbook of Lipid Research. DJ Hanahan (ed). New York, Plenum Press, 1983, pp 37-39
van Erpecum KJ, Carey MC: Influence of bile salts on molecular interactions between sphingomyelin and cholesterol: relevance to bile formation and stability. Biochim Biophys Acta 1345:269-282, 1997
Turley SD, Dietschy JM: Reevaluation of the 3α-hydroxysteroid dehydrogenase assay for total bile acids in bile. J Lipid Res 19:924-928, 1978
Rouser G, Fleischer S, Yamamoto A: Two dimensional thin layer chromatographic separation of polar lipids and determination of phospholipids by phosphorus analysis of spots. Lipids 5:494-496, 1970
Marra F, Gentilini A, Pinzani M, Choudhury GG, Parola M, Herbst H, Dianzani MU, Laffi G, Abboud HE, Gentilini, P: Phosphatidylinositol 3-kinase is required for platelet-derived growth factor's actions on hepatic stellate cells. Gastroenterology 112:1297-1306, 1997
Bradford MM: A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248-254, 1976
Duke RC, Cohen JJ, Chervenak R: Differences in target cell DNA fragmentation induced by mouse cytotoxic T lymphocytes and natural killer cells. J Immunol 137:1442-1447, 1986
Malina HZ, Richter C, Mehl M, Hess OM: Pathological apoptosis by xanthurenic acid, a tryptophan metabolite: activation of cell caspases but not cytoskeleton breakdown. BMC Physiol 1:7, 2001
Laemmli UK: Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685, 1970
Robino G, Parola M, Marra F, Caligiuri A, De Franco RM, Zamara E, Bellomo G, Gentilini P, Pinzani M, Dianzani MU: Interaction between 4-hydroxy-2,3-alkenals and the platelet-derived growth factor-beta receptor. Reduced tyrosine phosphorylation and downstream signaling in hepatic stellate cells. J Biol Chem 275:40561-40567, 2000
LaRue JM, Stratagoules ED, Martinez JD: Deoxycholic acid-induced apoptosis is switched to necrosis by bcl-2 and calphostin C. Cancer Lett 152:107-113, 2000
Schlottman K, Wachs FP, Krieg RC, Kullmann F, Scholmerich J, Rogler G: Characterization of bile salt-induced apoptosis in colon cancer cell lines. Cancer Res 60:4270-4276, 2000
Peiffer LP, Peters DJ, McGarrity TJ: Differential effects of deoxycholic acid on proliferation of neoplastic and differentiated colonocytes in vitro. Dig Dis Sci 42:2234-2240, 1997
Glinghammar B, Inoue H, Rafter JJ: Deoxycholic acid causes DNA damage in colonic cells with subsequent induction of caspases, COX-2 promoter activity and the transcription factors NF-kB and AP-1. Carcinogenesis 23:839-845, 2002
Crowley-Weber CL, Payne CM, Gleason-Guzman M, Watts GS, Futscher B, Waltmire CN, Crowley C, Dvorakova K, Bernstein C, Craven M, Garewal H, Bernstein H: Development and molecular characterization of HCT-116 cell lines resistant to the tumor promoter and multiple stress-inducer, deoxycholate. Carcinogenesis 23:2063-2080, 2002
Hannun YA, Linardic CM: Sphingolipid breakdown products: anti-proliferative and tumor-suppressor lipids. Biochim Biophys Acta 1154:223-236, 1993
Yedgar S, Gatt S: Enzymic hydrolysis of sphingomyelin in the presence of bile salts. Biochem J 185:749-754, 1980
Donovan JM, Jackson AA, Carey MC: Molecular species composition of intermixed micellar/vesicular bile salt concentrations in model bile: dependence upon hydrophilic-hydrophobic balance. J Lipid Res 34:1131-1140, 1993
Koynova R, Caffrey M: Phases and phase transitions of the sphingolipids. Biochim Biophys Acta 1255:213-236, 1995
Eckhardt ER, Wang DQ, Donovan JM, Carey MC: Dietary sphingomyelin suppresses intestinal cholesterol absorption by decreasing thermodynamic activity of cholesterol monomers. Gastroenterology 122:948-956, 2002
Nishioka T, Havinga R, Tazuma S, Stellaard F, Kuipers F, Verkade HJ: Enteral administration of phosphatidylcholine-cholesterol liposomes partially overcomes intestinal fat malabsorption in bile-deficient rats. Gastroenterology 4:A572, 2000
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Moschetta, A., Portincasa, P., van Erpecum, K. et al. Sphingomyelin Protects Against Apoptosis and Hyperproliferation Induced by Deoxycholate: Potential Implications for Colon Cancer. Dig Dis Sci 48, 1094–1101 (2003). https://doi.org/10.1023/A:1023712712025
Issue Date:
DOI: https://doi.org/10.1023/A:1023712712025