Abstract
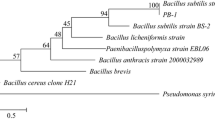
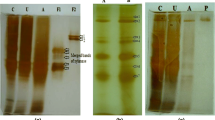
The gene for a novel enzyme having pectate lyase (Pel) and pectin methylesterase (Pme) activities found in the genome of an alkaliphilic Bacillus, KSM-P358, was sequenced. The structural gene contained a long open reading frame of 4314 bp corresponding to a 32-amino-acid signal peptide and a 1406-amino-acid mature enzyme with a molecular mass of 155,666. The mature enzyme contained two uncontiguous regions at amino acids 800–1051 and 1105–1406 exhibiting homology to a Pel from a Bacillus strain with 43.7% and a Pme from Erwinia chrysanthemi with 33.4% identity, respectively. The recombinant enzyme expressed in Bacillus subtilis cells had a molecular mass of 160 kDa and exhibited pH and temperature optima for Pel activity of 10 and 40 °C and those for the Pme activity of 8.5 and 45 °C. The genes for the domains for the Pel and Pme could be separately expressed in Escherichia coli cells, and the catalytic properties of the respective protein fragments were essentially identical to those of the intact enzyme. This novel enzyme is ‘mosaic’ in that some regions before the two domains exhibited limited but substantial similarity to some regions of carbohydrate-active enzymes. The regions contained parts of a gene for Pels from a Bacillus sp. and Pseudomonas fluorescens, a xylanase from P. fluorescens subsp. cellulosa, a 1,4-β-mannanase from a Pyromyces sp., a putative Pel from a Streptomyces coelicolor cosmid, a (1,3-1,4)-β-glucanase from Clostridium thermocellum.
Similar content being viewed by others
References
Barras, F., van Gijsegem, F. & Chatterjee, A.K. 1994 Extracellular enzymes and pathogenesis of soft-rot Erwinia. Annual Review of Phytopathology 32, 201–234.
Bekri, M.A., Desair, J., Keijers, V., Proost, P., Leeuwen, M.S., Vanderleyden, J. & Broek, V. 1999 Azospirillum irakense produces a novel type pectate lyase. Journal of Bacteriology 181, 2440–2447.
Chang, S. & Cohen, S.N. 1979 High frequency transformation of Bacillus subtilis protoplasts by plasmid DNA. Molecular and General Genetics 168, 111–115.
Copley, R.R. & Bork, P. 2000 Homology among (??)8 barrels: implication for the evolution of metabolic pathways. Journal of Molecular Biology 303, 627–641.
Coutinho, P.M. & Henrissat, B. 1999 Carbohydrate-active enzymes: an integrated database approach. In: Recent Advances in Carbohydrate Bioengineering, eds. Gilbert, H.J., Davies, G.J., Henrissat, B. & Svensson, B. pp. 3–12. Cambridge: Royal Society of Chemistry. ISBN 0-85404774-3.
Delincee, H. & Radola, B.J. 1970 Some size and charge properties of tomato pectin methylesterase. Biochimica et Biophysica Acta 214, 178–189.
Farber, G.K. & Petsko, G.A. 1990 The evolution of ?/? enzymes. Trends in Biochemical Sciences 15, 228–234.
Flint, H.J., Martin, J., Mcpherson, C.A., Daniel, A.S. & Zhang, J.-X. 1993 A bifunctional enzyme, with separate xylanase and ?(1,3-1,4)-glucanase domains, encoded by the xynD gene of Ruminococcus flavefaciens. Journal of Bacteriology 175, 2943–2951.
Gerlt, J.A. & Babbitt, P.C. 2001 Barrels in pieces? Nature Structural Biology 8, 5–7.
Gilbert, H.J., Hall, J., Hazlewood, G.P. & Ferreira, L.M.A. 1990 The N-terminal region of an endoglucanase from Pseudomonas fluorescens subspecies cellulosa constitutes a cellulose-binding domain that is distinct from the catalytic centre. Molecular Microbiology 4, 759–767.
Hanahan, D. 1983 Studies on transformation of Escherichia coli with plasmids. Journal of Molecular Biology 166, 557–580.
Hatada, Y., Igarashi, K., Ozaki, K., Ara, K., Hitomi, J., Kobayashi, T., Kawai, S., Watabe, T., & Ito, S. 1996 Amino acid sequence and molecular structure of an alkaline amylopullulanase from Bacillus that hydrolyzes ?-1,4 and ?-1,6 linkages in polysaccharides at different active sites. Journal of Biological Chemistry 271, 24,075–24,083.
Hatada, Y., Saito, K., Koike, K., Yoshimatsu, T., Ozawa, T., Kobayashi, T. & Ito, S. 2000 Deduced amino-acid sequence and possible catalytic residues of a novel pectate lyase from an alkaliphilic strain of Bacillus. European Journal of Biochemistry 267, 2268–2275.
Henrissat, B., Heffron, S.E., Yoder, M.D., Lietzke, S.E. & Jurnak, F. 1995 Functional implications of structure-based sequence alignment of proteins in the extracellular pectate lyase superfamily. Plant Physiology 107, 963–976.
Jenkins, J., Mayans, O., Smith, D., Worboys, K. & Pickersgill, R.W. 2001 Three-dimensional structure of Erwinia chrysanthemi pectin methylesterase reveals a novel esterase active site. Journal of Molecular Biology 305, 951–960.
Kellett, L.E., Poole, D.M., Ferreira, L.M.A., Durrant, A.J., Hazlewood, G.P. & Gilbert, H.J. 1990 Xylanase B and an arabinofuranosidase from Pseudomonas fluorescens subsp. cellulosa contain identical cellulose-binding domains and are encoded by adjacent genes. Biochemical Journal 272, 369–376.
Kita, N., Boyd, C.M., Garrett, M.R., Jurnak, F. & Keen, N.T. 1996 Differential effect of site-directed mutations in pelC on pectate lyase activity, plant tissue maceration, and elicitor activity. Journal of Biological Chemistry 271, 26,529–26,535.
Kobayashi, T., Koike, K., Yoshimatsu, T., Higaki, N., Suzumatsu, A., Ozawa, T., Hatada, Y. & Ito, S. 1999a Purification and properties of a low-molecular-weight, high-alkaline pectate lyase from an alkaliphilic strain of Bacillus. Bioscience Biotechnology and Biochemistry 63, 65–72.
Kobayashi, T., Hatada, Y., Higaki, N., Lusterio, D.D., Ozawa, T., Koike, K., Kawai, S. & Ito, S. 1999b Enzymatic properties and deduced amino acid sequence of a high-alkaline pectate lyase from an alkaliphilic Bacillus isolate. Biochimica et Biophysica Acta 1427, 145–154.
Kobayashi, T., Hatada, Y., Suzumatsu, A., Saeki, K., Hakamada, Y. & Ito, S. 2000 Highly alkaline pectate lyase Pel-4A from alkaliphilic Bacillus sp. strain P-4-N: its catalytic properties and deduced amino acid sequence. Extremophiles 4, 377–383.
Laemmli, U.K. 1970 Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685.
Laurent, F., Kotoujansky, A. & Bertheau, Y. 2000 Overproduction in Escherichia coli of the pectin methylesterase A from Erwinia chrysanthemi 3937: one-step purification, biochemical characterization, and production of polyclonal antibodies. Canadian Journal of Microbiology 46, 474–480.
Lietzke, S.E., Yoder, M.D., Keen, N.T. & Jurnak, F. 1994 The three-dimensional structure of pectate lyase E, a plant virulence factor from Erwinia chrysanthemi. Plant Physiology 106, 849–862.
Ogawa, A., Sawada, K., Saito, K., Hakamada, Y., Sumitomo, N., Hatada, Y., Kobayashi, T. & Ito, S. 2000 A new high-alkaline and high-molecular-weight pectate lyase from a Bacillus isolate: enzymatic properties and cloning of the gene for the enzyme. Bioscience Biotechnology and Biochemistry 64, 1133–1141.
Pickersgill, R., Jenkins, J., Harris, G., Nasser, W. & Robert-Baudouy, J. 1994 The structure of Bacillus subtilis pectate lyase in complex with calcium. Nature Structural Biology 1, 717–723.
Reardon, D. & Farber, G.K. 1995 The structure and evolution of ?/? barrel proteins. FASEB Journal 9, 497–503.
Rombouts, F.M. & Pilnik, W. 1980 Pectic enzymes. In Economic Microbiology, ed. Rose, A.H. vol. 5, pp. 227–282. London: Academic Press. ISBN 0-12-596555-9.
Saito, H. & Miura, K. 1963 Preparation of transforming deoxyribonucleic acid by phenol treatment. Biochimica et Biophysica Acta 72, 619–629.
Saitou, N. & Nei, M. 1987 A neighbor-joining method: a new method for reconstructing phylogenetic trees. Molecular Biology Evolution 44, 406–425.
Sakai, T., Sakamoto, T., Hallaert, J. & Vandamme, E.J. 1993 Pectin, pectinase, and protopectinase: production, properties, and applications. Advances in Applied Microbiology 39, 213–294.
Saul, D.J., Williams, L.C., Grayling, R.A., Chamley, L.W., Love, D.R. & Bergquist, P.L. 1990 celB, a gene coding for a bifunctional cellulase from the extreme thermophile 'Caldocellum accharoyticum'. Applied and Environmental Microbiology 56, 3117–3124.
Sawada, K., Ogawa, A., Ozawa, T., Sumitomo, N., Hatada, Y., Kobayashi, T. & Ito, S. 2000 Nucleotide and amino-acid sequences of a new-type pectate lyase from alkaliphilic strain of Bacillus. European Journal of Biochemistry 267, 1510–1515.
Scavetta, R.D., Herron, S.R., Hotchkiss, A.T., Kita, N., Keen, N.T., Benen, J.A.E., Kester, H.C.M., Visser, J. & Jurnak. F. 1999 Structure of a plant cell wall fragment complexed to pectate lyase C. Plant Cell 11, 1081–1092.
Takao, M., Nakaniwa, T., Yoshikawa, K., Terashita, T. & Sakai, T. 2000 Purification and characterization of thermostable pectate lyase with protopectinase activity from thermophilic Bacillus sp. TS 47. Bioscience Biotechnology and Biochemistry 64, 2360–2367.
Tardy, F., Nasser, W., Robert-Baudouy, J. & Hugouvieux-Cotte-Pattat, N. 1997 Comparative analysis of the five major Erwinia chrysanthemi pectate lyases: enzyme characteristics and potential inhibitors. Journal of Bacteriology 179, 2503–2511.
Tinoco, I.Jr, Borer, P.N., Dengler, B., Levine, M.D., Uhlenbeck, O.C., Crothers, D.M. & Gralla, J. 1973 Improved estimation of secondary structure of ribonucleic acids. Nature New Biology 246, 40–41.
Tjalsma, H., Bolhuis, A., Jongbloed, J.D.H., Bron, S. & van Dijl, J.M. 2000 Signal peptide-dependent protein transport in Bacillus subtilis: a genome-based survey of the secretome. Microbiology and Molecular Biology Reviews 64, 515–547.
Zverlov, V., Mahr, S., Riedel, K. & Bronnenmeier, K. 1998 Properties and gene structure of a bifunctional cellulolytic enzyme (CelA) from the extreme thermophile 'Anaerocellum thermophilum' with separate glycosyl hydrolase family 9 and 48 catalytic domains. Microbiology 144, 457–465.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kobayashi, T., Sawada, K., Sumitomo, N. et al. Bifunctional pectinolytic enzyme with separate pectate lyase and pectin methylesterase domains from an alkaliphilic Bacillus . World Journal of Microbiology and Biotechnology 19, 269–277 (2003). https://doi.org/10.1023/A:1023698007103
Issue Date:
DOI: https://doi.org/10.1023/A:1023698007103