Abstract
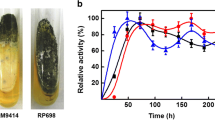
A total of 26 thermophilic isolates, selected from a compost of agricultural waste, which was mostly composed of vegetable, corncob and rice straw, were cultivated at 50 °C for further studies of thermostable cellulase production. The thermostable cellulase gene from the chromosomal DNA of actinomycetes isolate no. 10 was shotgun-cloned and transformed into Streptomyces sp. IAF 10-164. A transformant, T3-1, was found to be a good strain for the production of thermostable cellulases. Cultivation of T3-1 in modified Mandels–Reese broth containing 1% carboxymethylcellulose (CMC)-sodium salt and the optimal condition for microbial growth were studied. Batch cultivation in a flask revealed that CMCase and Avicelase production reached the maximum between the third to fifth day, whereas maximum β-glucosidase production occurred on the ninth day. Microbial biomass increased from the first day to the fifth day and then decreased. The crude enzyme had the highest activity at 50 °C and at pH 6.5. The enzyme was shown to be a thermostable cellulase whose activities were stable at 50 °C for more than 7 days.
Similar content being viewed by others
References
Beguin, P. & Aubert, J.P. 1994 The biological degradation of cellulose. FEMS Microbiology Reviews 13, 25–58.
Bhat, M.K. 2000 Cellulases and related enzymes in biotechnology. Biotechnology Advances 18, 355–383.
Bhat, M.K. & Bhat, S. 1997 Cellulose degrading enzymes and their potential industrial applications. Biotechnology Advances 15, 583–620.
Cheng, C.W., Lin, J.S., Liu, Y.T. & Yang, S.S. 2000 Cloning and expression of the ?-amylase gene and oxytetracycline production in Streptomyces rimosus. World Journal of Microbiology and Biotechnology 16, 225–230.
Fernandez-Abalos, J.M., Sanchez, P., Coll, P.M., Villanueva, J.R., Perez, P. & Santamaria, R.I. 1992 Cloning and nucleotide sequence of celA1 and endo-?-1,4-glucanase-encoding gene from Streptomyces halstedii JM8. Journal of Bacteriology 174, 6368–6376.
George, S.P., Ahmad, A. & Rao, M.B. 2001 Studies on carboxymethyl cellulase produced by an alkalothermophilic actinomycete. Bioresource Technology 77, 171–175.
Ghose, T.K. 1987 Measurement of cellulase activities. Pure and Applied Chemistry 52, 257–268.
Gomes, D.J., Gomes, J. & Steiner, W. 1994 Production of highly thermostable xylanase by a wild strain of thermophilic fungus Thermoascus aurantiacus and partial characterization of the enzyme. Journal of Biotechnology 37, 11–22.
Gomes, I., Gomes, J., Gomes, D.J. & Steiner, W. 2000 Simultaneous production of high activities of thermostable endoglucanase and ?-glocosidase by the wild thermophilic fungus Thermoascus aurantiacus. Applied Microbiology and Biotechnology 53, 461–468.
Halldorsdottir, S., Thorolfsdottir, E.T., Spilliaert, R., Johansson, M., Thorbjarnardottir, S.H., Palsdottir, A., Hreggvidsson, G.O., Kristjansson, J., Holst, O. & Eggertsson, G. 1998 Cloning, sequencing and overexpression of a Rhodothermus marinus gene encoding a thermostable cellulase of glycosyl hydrolase family 12. Applied Microbiology and Biotechnology 49, 277–284.
Hopwood, D.A., Bibb, M.J., Chater, K.F., Bruton, C.J., Kieser, H.M., Lydiate, D.J., Smith, C.P. & Ward, J.M. 1985 Genetic Manipulation Manipulation of Streptomyces: A Laboratory Manual. Norwich: The John Innes Foundation, ISBN 0-70840336-0.
Huck, T., Porter, A. & Bushell, M.E. 1991 Positive selection of antibiotic-producing soil isolates. Journal of General Microbiology 137, 2321–2329.
Kubicek, C.P. 1992 The cellulase protein of Trichoderma reesei: structure, multiplicity, mode of action and regulation of formation. Advances in Biochemical Engineering Biotechnology 45, 1–27.
Mandels, M. 1985 Applications of cellulases. Biochemical Society Transactions 13, 414–415.
Mandels, M., Medeiros, J.E., Andreotti, R.E. & Bissett, F.H. 1981 Enzymatic hydrolysis of cellulose: Evaluation of cellulase culture filtrates under the use conditions. Biotechnology Bioengineering 23, 2009–2026.
Meinke, A., Braun, C., Gilkes, N.R., Kilburn, D.G., Miller, R.C. Jr. & Warren, R.A.J. 1991 Unusual sequence organization in Cen B, an inverting endoglucanase from Cellulomonas fimi. Journal of Bacteriology 171, 308–314.
Merivuori, H., Siegler, K.M., Sands, J.A. & Montenecourt, B.S. 1984 Regulation of cellulase biosynthesis and secretion in fungi. Biochemical Society Transactions 13, 411–414.
Messner, R. & Kubicek, C.P. 1991 Carbon source control of cellobiohydrolase I and II formation by Trichoderma reesei. Applied and Environmental Microbiology 57, 630–635.
Miller, G.L. 1959 Use of dinitrosalicyclic acid reagent for determination of reducing sugar. Analytical Chemistry 31, 426–428.
Sanchez-Torres, J., Perez, P. & Santamaria, R.I. 1996 A cellulase gene from a new alkalophilic Bacillus sp. (strain N186-1). Its cloning, nucleotide sequence and expression in Escherichia coli. Applied Microbiology and Biotechnology 46, 149–155.
Schrempf, H. & Walter, S. 1995 The cellulolytic system of Streptomyces reticuli. International Journal of Biological Macromolecules 17, 353–355.
Thambirajah, J.J., Zulkali, M.D. & Hashim, M.A. 1995 Microbiological and biochemical changes during the composting of oil palm empty-fruit-bunches. Effect of nitrogen supplementation on the substrates. Bioresource Technology 52, 133–144.
Theberge, M., Lacaze, P., Shereck, F., Morosol, R. & Kluepfel, D. 1992 Purification and characterization of an endoglucanase from Streptomyces lividans 66 and DNA sequence of the gene. Applied and Environmental Microbiology 58, 815–820.
Tomme, P., Van Tilbeugh, H., Pettersson, G. & Van Damme, M. 1988 Studies of the cellulolytic system of Trichoderma reesei QM9414. Analysis of domain function in two cellobiohydrolases by limited proteolysis. European Journal of Biochemistry 170, 575–581.
Wang, W., Reid, S.J. & Thomson, J.A. 1993 Transcription regulation of an endoglucanase and a cellodextrinase gene in Ruminococcus flavefaciens FD-1. Journal of General Microbiology 139, 1219–1226.
Wittmann, S., Shareck, F., Kluepfel, D. & Morosol, R. 1994 Purification and characterization of the CelB endoglucanase from Streptomyces lividans 66 and DNA sequence of the encoding gene. Applied and Environmental Microbiology 60, 1701–1703.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Jang, HD., Chen, KS. Production and characterization of thermostable cellulases from Streptomyces transformant T3-1. World Journal of Microbiology and Biotechnology 19, 263–268 (2003). https://doi.org/10.1023/A:1023641806194
Issue Date:
DOI: https://doi.org/10.1023/A:1023641806194