Abstract
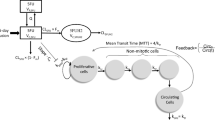
As myelosuppression is the dose-limiting toxicity for most chemotherapeutic drugs, modelers attempt to find relationships between drug and toxicity to optimize treatment. Mechanistic models, i.e. models based on physiology and pharmacology, are preferable over empirical models, as prior information can be utilized and as they generally are more reliable for extrapolations. To account for different dosing-regimens and possible schedule-dependent effects, the whole concentration–time profile should be used as input into the pharmacokinetic–pharmacodynamic model. It is also of importance to model the whole time course of myelosuppression to be able to predict both the degree and duration of toxicity as well as consecutive courses of therapy. A handful of (semi)-mechanistic pharmacokinetic–pharmacodynamic models with the above properties have been developed and are reviewed. Ideally, a model of myelosuppression should separate drug-specific parameters from system related parameters to be applicable across drugs and useful under different clinical settings. Introduction of mechanistic models of myelosuppression in the design and evaluation of clinical trials can guide in the decision of optimal sampling times, contribute to knowledge of optimal doses and treatment regimens at an earlier time point and identify sub-groups of patients at a high risk of myelosuppression.
Similar content being viewed by others
References
Bodey GP, Buckley M, Sathe YS, Freireich EJ: Quantitative relationships between circulating leukocytes and infection in patients with acute leukemia. Ann Intern Med 64: 328–340, 1966
Minami H, Sasaki Y, Watanabe T, Ogawa M: Pharmacodynamic modeling of the entire time course of leukopenia after a 3–hour infusion of paclitaxel. Jpn J Cancer Res 92: 231–238, 2001
Stoller RG, Hande KR, Jacobs SA, Rosenberg SA, Chabner BA: Use of plasma pharmacokinetics to predict and prevent methotrexate toxicity. N Engl J Med 297: 630–634, 1977
Evans WE, Relling MV, Rodman JH, Crom WR, Boyett JM, Pui CH: Conventional compared with individualized chemotherapy for childhood acute lymphoblastic leukemia. N Engl J Med 338: 499–505, 1998
Ratain MJ: Therapeutic relevance of pharmacokinetics and pharmacodynamics. Semin Oncol 19: 8–13, 1992
Egorin MJ, Van Echo DA, Whitacre MY, Forrest A, Sigman LM, Engisch KL, Aisner J: Human pharmacokinetics, excretion, and metabolism of the anthracycline antibiotic menogaril (7–OMEN, NSC 269148) and their correlation with clinical toxicities. Cancer Res 46: 1513–1520, 1986
Trump DL, Egorin MJ, Forrest A, Willson JK, Remick S, Tutsch KD: Pharmacokinetic and pharmacodynamic analysis of fluorouracil during 72–hour continuous infusion with and without dipyridamole. J Clin Oncol 9: 2027–2035, 1991
Gianni L, Kearns CM, Giani A, Capri G, Vigano L, Lacatelli A, Bonadonna G, Egorin MJ: Nonlinear pharmacokinetics and metabolism of paclitaxel and its pharmacokinetic/pharmacodynamic relationships in humans. J Clin Oncol 13: 180–190, 1995
Karlsson MO, Molnar V, Bergh J, Freijs A, Larsson R: A general model for time-dissociated pharmacokinetic-pharmacodynamic relationship exemplified by paclitaxel myelosuppression. Clin Pharmacol Ther 63: 11–25, 1998
Minami H, Sasaki Y, Saijo N, Ohtsu T, Fujii H, Igarashi T, Itoh K: Indirect-response model for the time course of leukopenia with anticancer drugs. Clin Pharmacol Ther 64: 511–521, 1998
Friberg LE, Brindley CJ, Karlsson MO, Devlin AJ: Models of schedule dependent haematological toxicity of 2′-deoxy-2′-methylidenecytidine (DMDC). Eur J Clin Pharmacol 56: 567–574, 2000
Lokich J, Anderson N: Dose intensity for bolus versus infusion chemotherapy administration: review of the literature for 27 anti-neoplastic agents. Ann Oncol 8: 15–25, 1997
Crawford J, Ozer H, Stoller R, Johnson D, Lyman G, Tabbara I, Kris M, Grous J, Picozzi V, Rausch G, Smith R, Gradishar W, Yahanda A, Vincent M, Stewart M, Glaspy J: Reduction by granulocyte colony-stimulating factor of fever and neutropenia induced by chemotherapy in patients with small-cell lung cancer. N Engl J Med 325: 164–170, 1991
Moore MJ, Theissen JJ: Cytotoxics and irreversible effects. In: van Boxtel CJ, Holford NHG, Danhof M (eds) The in vivo Study of Drug Action. Elsevier Science Publisher B.V., Amsterdam, 1992, pp 377–400
Rosner GL, Muller P: Pharmacodynamic analysis of hematologic profiles. J Pharmacokinet Biopharm 22: 499–524, 1994
Karlsson MO, Port RE, Ratain MJ, Sheiner LB: A population model for the leukopenic effect of etoposide. Clin Pharmacol Ther 57: 325–334, 1995
Friberg LE, Freijs A, Sandström M, Karlsson MO: Semiphysiological model for the time course of leukocytes after varying schedules of 5–fluorouracil in rats. J Pharmacol Exp Ther 295: 734–740, 2000
Zamboni WC, D'Argenio DZ, Stewart CF, MacVittie T, Delauter BJ, Farese AM, Potter DM, Kubat NM, Tubergen D, Egorin MJ: Pharmacodynamic model of topotecan-induced time course of neutropenia. Clin Cancer Res 7: 2301–2308, 2001
Friberg LE, Henningsson A, Maas H, Nguyen L, Karlsson MO: Model of chemotherapy-induced myelosuppression with parameter consistency across drugs. J Clin oncol 20: 4713–4721, 2002
Ackland SP, Ratain MJ, Vogelzang NJ, Choi KE, Ruane M, Sinkule JA: Pharmacokinetics and pharmacodynamics of long-term continuous-infusion doxorubicin. Clin Pharmacol Ther 45: 340–347, 1989
Miller AA, Tolley EA, Niell HB, Griffin JP, Mauer AM: Pharmacodynamics of prolonged oral etoposide in patients with advanced non-small-cell lung cancer. J Clin Oncol 11: 1179–1188, 1993
Ratain MJ, Schilsky RL, Choi KE, Guarnieri C, Grimmer D, Vogelzang NJ, Senekjian E, Liebner MA: Adaptive control of etoposide administration: impact of interpatient pharmacodynamic variability. Clin Pharmacol Ther 45: 226–233, 1989
Minami H, Ando Y, Sakai S, Shimokata K: Clinical and pharmacologic analysis of hyperfractionated daily oral etoposide. J Clin Oncol 13: 191–199, 1995
Jakobsen P, Bastholt L, Dalmark M, Pfeiffer P, Petersen D, Gjedde SB, Sandberg E, Rose C, Nielsen OS, Mouridsen HT: A randomized study of epirubicin at four different dose levels in advanced breast cancer. Feasibility of myelotoxicity prediction through single blood-sample measurement. Cancer Chemother Pharmacol 28: 465–469, 1991
van Groeningen CJ, Pinedo HM, Heddes J, Kok RM, de Jong APJM, Wattel E, Peters GJ, Lankelma J: Pharmacokinetics of 5–fluorouracil assessed with a sensitive mass spectrometric method in patients on a dose escalation schedule. Cancer Res 48: 6956–6961, 1988
Zhou H, Choi L, Lau H, Bruntsch U, Vries EE, Eckhardt G, Oosterom AT, Verweij J, Schran H, Barbet N, Linnartz R, Capdeville R: Population pharmacokinetics/toxicodynamics (PK/TD) relationship of SAM486A in phase I studies in patients with advanced cancers. J Clin Pharmacol 40: 275–283, 2000
Sheiner LB, Steimer JL: Pharmacokinetic/pharmacodynamic modeling in drug development. Annu Rev Pharmacol Toxicol 40: 67–95, 2000
Lee WM, Dang CV: Control of cell growth and differentiation. In: Hoffman R, Benz EJ, Sanford J, Shattil SJ, Furie B, Cohen HJ, Silberstein LE, McGlave P (eds) Hematology. Basic Principles and Practice, 3rd edn. Churchill Livingstone, New York, 1999, pp 57–71
Botnick LE, Hannon EC, Hellman S: Nature of the hemopoietic stem cell compartment and its proliferative potential. Blood Cells 5: 195–210, 1979
Lemischka IR, Raulet DH, Mulligan RC: Developmental potential and dynamic behavior of hematopoietic stem cells. Cell 45: 917–927, 1986
Maloney MA, Patt HM: Granulocyte transit from bone marrow to blood. Blood 31: 195–201, 1968
Finch CA, Harker LA, Cook JD: Kinetics of the formed elements of human blood. Blood 50: 699–707, 1977
Dancey JT, Deubelbeiss KA, Harker LA, Finch CA: Neutrophil kinetics in man. J Clin Invest 58: 705–715, 1976
Fliedner TM, Cronkite EP, Killmann SÅ, Bond VP: Granulocytopoiesis. II. Emergence and pattern of labeling of neutrophils granulocytes in humans. Blood 24: 683–700, 1964
Athens JW, Raab SO, Haab OP, Mauer AM, Aschenbrucker H, Cartwright GE, Wintrobe MM: Leukokinetic studies. III. The distribution of granulocytes in the blood of normal subjects. J Clin Invest 40: 159–164, 1961
Mauer AM, Athens JW, Aschenbrucker H, Cartwright GE, Wintrobe MM: Leukokinetic studies, II. A method for labeling granulocytes in vitro with radioactive diisopropylfluorophosphate (DFP32). J Clin Invest 39: 1481–1486, 1960
Cartwright GE, Athens JW, Wintrobe MM: The kinetics of granulopoiesis in normal man. Blood 24: 780–803, 1964
MacLennan ICM, Drayson MT: Normal lymphocytes and non-neoplastic lymphocyte disorders. In: Hoffbrand AV, Lewis SM, Tuddenham EGD (eds) Postgraduate Haematology, 4th edn. Butterworth Heinemann, Oxford, 1999, p. 296
Demetri GD, Griffin JD: Granulocyte colony-stimulating factor and its receptor. Blood 78: 2791–2808, 1991
Haurie C, Dale DC, Mackey MC: Cyclical neutropenia and other periodic hematological disorders: a review of mechanisms and mathematical models. Blood 92: 2629–2640, 1998
Verfaillie CM: Anatomy and physiology of hematopoiesis. In: Hoffman R, Benz EJ, Sanford J, Shattil SJ, Furie B, Cohen HJ, Silberstein LE, McGlave P (eds) Hematology. Basic Principles and Practice, 3rd edn. Churchill Livingstone, New York, 1999, pp 139–154
Lord BI, Bronchud MH, Owens S, Chang J, Howell A, Souza L, Dexter TM: The kinetics of human granulopoiesis following treatment with granulocyte colony-stimulating factor in vivo. Proc Natl Acad Sci USA 86: 9499–9503, 1989
Price TH, Chatta GS, Dale DC: Effect of recombinant granulocyte colony-stimulating factor on neutrophil kinetics in normal young and elderly humans. Blood 88: 335–340, 1996
Williams GT, Smith CA, Spooncer E, Dexter TM, Taylor DR: Haemopoietic colony stimulating factors promote cell survival by suppressing apoptosis. Nature 343: 76–79, 1990
Takatani H, Soda H, Fukuda M, Watanabe M, Kinoshita A, Nakamura T, Oka M: Levels of recombinant human granulocyte colony-stimulating factor in serum are inversely correlated with circulating neutrophil counts. Antimicrob Agents Chemother 40: 988–991, 1996
Ericson SG, Gao H, Gericke GH, Lewis LD: The role of polymorphonuclear neutrophils (PMNs) in clearance of granulocyte colony-stimulating factor (G-CSF) in vivo and in vitro. Exp Hematol 25: 1313–1325, 1997
Gupta P, Blazar BR, Gupta K, Verfaillie CM: Human CD34(+) bone marrow cells regulate stromal production of interleukin-6 and granulocyte colony-stimulating factor and increase the colony-stimulating activity of stroma. Blood 91: 3724–3733, 1998
Bronchud MH, Scarffe JH, Thatcher N, Crowther D, Souza LM, Alton NK, Testa NG, Dexter TM: Phase I/II study of recombinant human granulocyte colony-stimulating factor in patients receiving intensive chemotherapy for small cell lung cancer. Br J Cancer 56: 809–813, 1987
Suwa T, Hogg JC, English D, Van Eeden SF: Interleukin-6 induces demargination of intravascular neutrophils and shortens their transit in marrow. Am J Physiol Heart Circ Physiol 279: H2954–H2960, 2000
Selleri C, Sato T, Anderson S, Young NS, Maciejewski JP: Interferon-gamma and tumor necrosis factor-alpha suppress both early and late stages of hematopoiesis and induce programmed cell death. J Cell Physiol 165: 538–546, 1995
Harrison DE, Lerner CP: Most primitive hematopoietic stem cells are stimulated to cycle rapidly after treatment with 5–fluorouracil. Blood 78: 1237–1240, 1991
Tannock IF: Experimental chemotherapy and concepts related to the cell cycle. Int J Radiat Biol Relat Stud Phys Chem Med 49: 335–355, 1986
Sundman-Engberg B, Tidefelt U, Paul C: Toxicity of cytostatic drugs to normal bone marrow cells in vitro. Cancer Chemother Pharmacol 42: 17–23, 1998
Lajtha LG, Oliver R, Gurney CW: Kinetic model of a bone-marrow stem-cell population. Brit J Haematol 8: 442–460, 1962
Rubinow SI: A simple model of a steady state differentiating cell system. J Cell Biol 43: 32–39, 1969
King-Smith EA, Morley A: Computer simulation of granulopoiesis: normal and impaired granulopoiesis. Blood 36: 254–262, 1970
Rubinow SI, Lebowitz JL, Sapse AM: Parameterization of in vivo leukemic cell populations. Biophys J 11: 175–188, 1971
Blumenson LE: A comprehensive modeling procedure for the human granulopoietic system: Over-all view and summary of data. Blood 42: 303–313, 1973
Rubinow SI, Lebowitz JL: A mathematical model of neutrophil production and control in normal man. J Math Biol 1: 187–225, 1975
Wheldon: Mathematical models of oscillatory blood cell production. Math Biosci 24: 289–305, 1975
Rubinow SI, Lebowitz JL: A mathematical model of the chemotherapeutic treatment-of acute myeloblastic leukemia. Biophys J 16: 1257–1271, 1976
Blumenson LE, Bross ID: Assessment of myelotoxic effects of chemotherapy from early leukopenic response: application of a mathematical model for granulopoiesis. J Surg Oncol 11: 171–176, 1979
Smeby W, Benestad HB: Simulation of murine granulopoiesis. Blut 41: 47–60, 1980
Steinbach KH, Raffler H, Pabst G, Fliedner TM: A mathematical model of canine granulocytopoiesis. J Math Biol 10: 1–12, 1980
Wichmann HE, Loeffler M: Mathematical Modeling of Cell Proliferation: Stem Cell Regulation in Hemopoiesis, Vol 1. CRC Press, Boca Raton, 1985
Wichmann HE, Loeffler M, Schmitz S: A concept of hemopoietic regulation and its biomathematical realization. Blood Cells 14: 411–429, 1988
Fokas AS, Keller JB, Clarkson BD: Mathematical model of granulocytopoiesis and chronic myelogenous leukemia. Cancer Res 51: 2084–2091, 1991
Schmitz S, Franke H, Brusis J, Wichmann HE: Quantification of the cell kinetic effects of G-CSF using a model of human granulopoiesis. Exp Hematol 21: 755–760, 1993
Hearn T, Haurie C, Mackey MC: Cyclical neutropenia and the peripheral control of white blood cell production. J Theor Biol 192: 167–181, 1998
Haurie C, Dale DC, Rudnicki R, Mackey MC: Modeling complex neutrophil dynamics in the grey collie. J Theor Biol 204: 505–519, 2000
Kahn MG, Fagan LM, Sheiner LB: Combining physiologic models and symbolic methods to interpret time-varying patient data. Methods Inf Med 30: 167–178, 1991
Sun YN, Jusko WJ: Transit compartments versus gamma distribution function to model signal transduction processes in pharmacodynamics. J Pharm Sci 87: 732–737, 1998
Roskos LK, Cheung EN, Vincent M, Foot MA, Morstyn G: Pharmacology of filgrastim (r-metHuG-CSF). In: Morstyn G, Dexter TM, Foote MA (eds) Filgrastim (r-metHuG-CSF) in Clinical Practice. Marcel Dekker, New York, 1998, pp 51–71
Krzyzanski W, Ramakrishnan R, Jusko WJ: Basic pharmacodynamic models for agents that alter production of natural cells. J Pharmacokinet Biopharm 27: 467–489, 1999
Wang B, Ludden TM, Cheung EN, Schwab GG, Roskos LK: Population pharmacokinetic-pharmacodynamic modeling of filgastrim (r-metHuG-CSF) in healthy volunteers. J Pharmacokinet Pharmacodyn 28: 321–342, 2001
Westermann J, Puskas Z, Pabst R: Blood transit and recirculation kinetics of lymphocyte subsets in normal rats. Scand J Immunol 28: 203–210, 1988
Friberg LE, Karlsson MO: A semi-physiological model for the pharmacodynamic interaction on leukocytes after 5–fluorouracil and epirubicin administration (Abstract). 10th Population Approach Group Europe Meeting, Basel, 2001. http://userpage.fu-berlin.de/~page/
Hrushesky WJ, Bjarnason GA: Circadian cancer therapy. J Clin Oncol 11: 1403–1417, 1993
Sandström M, Lindman H, Bergh J, Karlsson MO: PK/PD of the epirubicin-docetaxel therapy in breast cancer patients (Abstract). 10th Population Approach Group Europe Meeting, Basel, 2001. http://userpage.fu-berlin.de/~page/
Bruno R, Hille D, Riva A, Vivier N, ten Bokkel Huinnink WW, van Oosterom AT, Kaye SB, Verweij J, Fossella FV, Valero V, Rigas JR, Seidman AD, Chevallier B, Fumoleau P, Burris HA, Ravdin PM, Sheiner LB: Population pharmacokinetics/pharmacodynamics of docetaxel in phase II studies in patients with cancer. J Clin Oncol 16: 187–196, 1998
Ando M, Minami H, Ando Y, Sakai S, Shimono Y, Sugiura S, Saka H, Shimokata K, Hasegawa Y: Pharmacological analysis of etoposide in elderly patients with lung cancer. Clin Cancer Res 5: 1690–1695, 1999
Ulich TR, del Castillo J: The hematopoietic and mature blood cells of the rat: their morphology and the kinetics of circulating leukocytes in control rats. Exp Hematol 19: 639–648, 1991
Collins JM, Zaharko DS, Dedrick RL, Chabner BA: Potential roles for preclinical pharmacology in phase I clinical trials. Cancer Treat Rep 70: 73–80, 1986
Collins JM, Grieshaber CK, Chabner BA: Pharmacologically guided phase I clinical trials based upon preclinical drug development. J Natl Cancer Inst 82: 1321–1326, 1990
Iliadis A, Barbolosi D: Optimizing drug regimens in cancer chemotherapy by an efficacy-toxicity mathematical model. Comput Biomed Res 33: 211–226, 2000
Barbolosi D, Iliadis A: Optimizing drug regimens in cancer chemotherapy: a simulation study using a PK-PD model. Comput Biol Med 31: 157–172, 2001
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Friberg, L.E., Karlsson, M.O. Mechanistic Models for Myelosuppression. Invest New Drugs 21, 183–194 (2003). https://doi.org/10.1023/A:1023573429626
Issue Date:
DOI: https://doi.org/10.1023/A:1023573429626