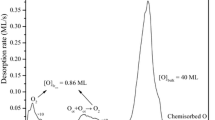
Abstract
Despite the structural similarities between methanol and methyl mercaptan (CH3SH), replacing the oxygen atom by sulfur has a profound effect on their chemistry at copper surfaces. In a combined STM, XPS and TPD study of the reaction of methyl mercaptan with clean and partially oxidised Cu(110) surfaces we have found that unlike methanol, the scission of the SH bond (both in the presence of and in the absence of oxygen) to give adsorbed mercaptide (CH3S(a)) results in a restructuring of the surface. Low concentrations of adsorbed mercaptide (< 1 × 1014 cm-2) result in a severe degradation of the STM image due to the high mobility of the surface adlayer. A stable surface can be regained by increasing the concentration of mercaptide or the presence of another adsorbate such as oxygen. The reconstructed surface is characterised by very narrow terraces (typically 10-15Å wide) orientated mainly in the 〈1¯10〉 direction with a “zig-zag” structure. Higher resolution images of the terraces reveal an atomic scale structure with a c(2 × 2) unit cell, each cell containing (in total) two bright features. The XPS data confirm that mercaptide is present and show that the concentration at which islands of mercaptide become visible in the STM images is approximately 3 × 1014 cm-2. At a surface concentration of 5.1 × 1014 cm-2 the c(2 × 2) structure is seen to be complete, consistent with the c(2 × 2) unit cell containing two mercaptide species. On heating to 450K the mercaptide dissociates to give chemisorbed sulfur adatoms and methane. The latter implies that the hydrogen formed when the methyl mercaptan dissociates remains chemisorbed at the surface until removed by reaction with the methyl groups. At pre-oxidised surfaces where chemisorbed hydrogen is removed as water, mercaptide decomposition leads to ethane desorption with minor methane and ethane components, the latter indicating that methyl dehydrogenation is possible at the copper surface. STM shows that following the decomposition of the mercaptide adlayer the copper surface regains its original structure of broad terraces (typically 100-200Å wide), although a monolayer of chemisorbed sulfur is now present.
Oxygen chemisorption is completely inhibited by mercaptide concentrations of 5 × 1014 cm-2 but occurs at lower concentrations. The STM images show that the mercaptide and oxygen adsorbates form separate islands, in contrast to the coadsorption of hydrogen sulfide and oxygen. The influence of oxygen on the thermal desorption of mercaptide is discussed in the light of the structural data.
Similar content being viewed by others
References
A.F. Carley, P.R. Davies, R.V. Jones, K.R. Harikumar, G.U. Kulkarni and M.W. Roberts, Surf. Sci. 447 (2000) 39.
A.F. Carley and M.W. Roberts, Proc. Roy. Soc. Lond. A 363 (1978) 403.
J.H. Scofield, J. Elec. Spec. Rel. Phen. 8 (1976) 129.
T.E. Madey, J.T. YatesJr and N.E. Erickson, Chem. Phys. Lett. 19 (1973) 487.
R.F. Reilman, A. Msezane and S.T. Manson, J. Elec. Spec. Rel. Phen. 8 (1976) 389.
B.A. Sexton and G.L. Nyberg, Surf. Sci. 165 (1986) 251.
P.W. Murray, F. Besenbacher and I. Stensgaard, Israel J. Chem. 36 (1996) 25.
F.M. Leibsle, S.M. Francis, S. Haq and M. Bowker, Surf. Sci. 318 (1994) 46.
C.T. Au, A.F. Carley and M.W. Roberts, Phil. Trans. Roy. Soc. A 318 (1986) 61.
M.W. Roberts, Chem. Soc. Rev. 18 (1989) 451.
M.W. Roberts, Surf. Sci. 300 (1994) 769.
A.F. Carley, P.R. Davies, M.W. Roberts and D. Vincent, Top. Catal. 1 (1994) 35.
X.C. Guo and R.J. Madix, Faraday Discuss. (1996) 139.
A.F. Carley, P.R. Davies, R.V. Jones, K.R. Harikumar, G.U. Kulkarni and M.W. Roberts, Top. Catal. 11 (2000) 299.
A.F. Carley, P.R. Davies, R.V. Jones, K.R. Harikumar and M.W. Roberts, Chem. Commun. (2000) 185.
A.F. Carley, P.R. Davies, P.H. Griffiths, M.W. Roberts, B.P. Williams and N. Young (in preparation).
F. Jensen, F. Besenbacher, E. Laesgaard and I. Stensgaard, Phys. Rev. B: Cond. Mat. 41 (1990) 10233.
A.F. Carley, P.R. Davies, K.R. Harikumar, R.V. Jones and M.W. Roberts (in preparation).
L. Wilde, N. Pangher and J. Haase, Surf. Sci. 347 (1996) 33.
D.T. Vu and K.A.R. Mitchell, Phys. Rev. B: Cond. Mat. 49 (1994) 11515.
M. Kostelitz and J. Oudar, Surf. Sci. 27 (1971) 176.
I. Stensgaard, L. Ruan, F. Besenbacher, F. Jensen and E. Laegsgaard, Surf. Sci. 270 (1992) 81.
S.M. Driver and D.P. Woodruff, Surf. Sci. 457 (2000) 11.
M.S. Kariapper, G.F. Grom, G.J. Jackson, C.F. McConville and D.P. Woodruff, J. Phys. Conden. Mat. 10 (1998) 8661.
A. Imanishi, S. Takenaka, T. Yokoyama, Y. Kitajima and T. Ohta, J. De Physique IV 7 (1997) 701.
R.W.G. Wykoff, Crystal Structures (Interscience, London, 1948).
A.F. Wells, Structural Inorganic Chemistry, 4th edn (Oxford, UK, 1975).
R. McGrath, A.A. Macdowell, T. Hashizume, F. Sette and P.H. Citrin, Phys. Rev. Lett. 64 (1990) 575.
C.C. Bahr, J.J. Barton, Z. Hussain, S.W. Robey, J.G. Tobin and D.A. Shirley, Phys. Rev. B: Cond. Mat. 35 (1987) 3773.
H.C. Zeng, R.N.S. Sodhi and K.A.R. Mitchell, Surf. Sci. 177 (1986) 329.
N.P. Prince, D.L. Seymour, M.J. Ash win, C.F. McConville, D.P. Woodruff and R.G. Jones, Surf. Sci. 230 (1990) 13.
A. Atrei, A.L. Johnson and D.A. King, Surf. Sci. 254 (1991) 65.
A. Imanishi, K. Isawa, F. Matsui, T. Tsuduki, T. Yokoyama, H. Kondoh, Y. Kitajima and T. Ohta, Surf. Sci. 407 (1998) 282.
A.F. Carley, P.R. Davies and M.W. Roberts, Chem. Commun. (1998) 1793.
P.R. Davies and G.G. Mariotti, Chem. Commun. (1996) 2319.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Carley, A.F., Davies, P.R., Jones, R.V. et al. A Combined XPS/STM and TPD Study of the Chemisorption and Reactions of Methyl Mercaptan at a Cu(110) Surface. Topics in Catalysis 22, 161–172 (2003). https://doi.org/10.1023/A:1023559516485
Issue Date:
DOI: https://doi.org/10.1023/A:1023559516485