Abstract
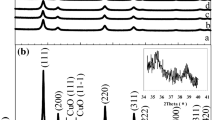
A series of Ce1-xCuxO2-δ mixed oxides were synthesized using a co-precipitation method and tested as catalysts for the steam reforming of methanol. XRD patterns of the Ce1-xCuxO2-δ mixed oxides indicated that Cu2+ ions were dissolved in CeO2 lattices to form a solid solution by calcination at 773K when x < 0.2. A TPR (temperature-programmed reduction) investigation showed that the CeO2 promotes the reduction of the Cu2+ species. Two reduction peaks were observed in the TPR profiles, which suggested that there were two different Cu2+ species in the Ce1-xCuxO2-δ mixed oxides. The TPR peak at low temperature is attributed to the bulk Cu2+ species which dissolved into the CeO2 lattices, and the peak at high temperature is due to the CuO species dispersed on the surface of CeO2. The Ce1-xCuxO2-δ mixed oxides were reduced to form Cu/CeO2 catalysts for steam reforming of methanol, and were compared with Cu/ZnO, Cu/Zn(Al)O and Cu/AL2O3 catalysts. All the Cu-containing catalysts tested in this study showed high selectivities to CO2 (over 97%) and H2. A 3.8wt% Cu/CeO2 catalyst showed a conversion of 53.9% for the steam reforming of methanol at 513K (W/F = 4.9 g h mol-1), which was higher than that over Cu/ZnO (37.9%), Cu/Zn(Al)O (32.3%) and Cu/AL2O3 (11.2%) with the same Cu loading under the same reaction conditions. It is likely that the high activity of the Cu/CeO2 catalysts may be due to the highly dispersed Cu metal particles and the strong metalsupport interaction between the Cu metal and CeO2 support. Slow deactivations were observed over the 3.8wt% Cu/CeO2 catalyst at 493 and 513K. The activity of the deactivated catalysts can be regenerated by calcination in air at 773K followed by reduction in H2 at 673K, which indicated that a carbonaceous deposit on the catalyst surface caused the catalyst deactivation. Using the TPO (temperature-programmed oxidation) method, the amounts of coke on the 3.8wt% Cu/CeO2 catalyst were 0.8wt% at 493K and 1.7wt% at 513K after 24h on stream.
Similar content being viewed by others
References
J.C. Amphlett, M.J. Evans, R.A. Jones, R.F. Mann and R.D. Weir, Can. J. Chem. Eng. 59 (1981) 720.
G. Shen, S. Fujita, S. Matsumoto and N. Takezawa, J. Mol. Catal. A: Chem. 124 (1997) 123.
C.J. Jiang, D.L. Trimm and M.S. Wainwright, Appl. Catal. A: Gen. 97 (1993) 145.
B.A. Peppley, J.C. Amphlett, L.M. Kearns and R.F. Mann, Appl. Catal. A: Gen. 179 (1999) 21.
J.P. Breen and J.R.H. Ross, Catal. Today 51 (1999) 521.
A. Trovareli, C. Leitenburg and G. Dolcetti, Chemtech June (1997) 32.
M. Pijolat, M. Prin and M. Soustelle, J. Chem. Soc. Faraday Trans. 91 (1995) 3941.
P. Fornasiero, G. Balducci, R.D. Monte, J. Kaspar, V. Sergo, G. Gubitosa, A. Ferrero and M. Graziani, J. Catal. 164 (1996) 173.
L. Fan and K. Fujimoto, J. Catal. 172 (1997) 238.
W. Liu and M.F. Stephanopoulos, J. Catal. 153 (1995) 304.
A.M. Arias, R. Cataluna, J.C. Conesa and J. Soria, J. Phys. Chem. B 102 (1998) 809.
S. Hocevar, J. Batista and J. Levec, J. Catal. 184 (1999) 39.
J.A. Rodriguez, T. Jirsak, A. Freitag, J.C. Hanson, J.Z. Larese and S. Chaturredi, Catal. Lett. 62 (1999) 113.
S. Velu, K. Suzuki and T. Osaki, Catal. Lett. 62 (1999) 159.
J.F. Scholten and A. van Montfoort, J. Catal. 1 (1962) 85.
T. Inui, M. Suehiro and Y. Takegami, J. Japan Petrol. Inst. 25 (1982) 63.
R.J. Kokes and P.H. Emmett, J. Am. Chem. Soc. 82 (1960) 1037.
J.A. Dean, (ed.), in: Lang's Handbook of Chemistry, 23rd edn (McGraw-Hill, New York, 1985) p. 3.
B.E. Goodby and J.E. Pemberton, Appl. Spectrosc. 42 (1988) 754.
G.R. Sheffer and T.S. King, J. Catal. 116 (1989) 488.
G. Apai, J.R. Monnier and D.R. Preuss, J. Catal. 98 (1986) 563.
Z. Xu, Z. Qian, L. Mao, K. Tanabe and H. Hattori, Bull. Chem. Soc. Jpn. 64 (1991) 1658.
T. Fujitani, M. Saito, Y. Kanai, T. Kakumoto and T. Watanabe, Catal. Lett. 25 (1994) 271.
R.O. Idem and N.N. Bakhshi, Ind. Eng. Chem. Res. 33 (1994) 2056.
R. Shiozaki, A.G. Anderson, T. Hayakawa, K. Suzuki, M. Shimizu and K. Takehira, J. Chem. Soc. Faraday Trans. 93 (1997) 3235.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, Y., Hayakawa, T., Tsunoda, T. et al. Steam Reforming of Methanol Over Cu/CeO2 Catalysts Studied in Comparison with Cu/ZnO and Cu/Zn(Al)O Catalysts. Topics in Catalysis 22, 205–213 (2003). https://doi.org/10.1023/A:1023519802373
Issue Date:
DOI: https://doi.org/10.1023/A:1023519802373