Abstract
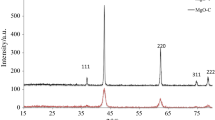
On calcination of basic magnesium carbonate at 20-800°C, an induction period was observed in the initial stage of thermal decomposition. At the decomposition degree higher than 20% of the weight loss, the specific surface area varied in proportion with the decomposition degree, which points to equal rates of gas liberation and recrystallization of intermediate products into an active oxide. The individual phase of magnesium oxide alone is identified at the minimal temperature of 500°C. As the calcination temperature and time increase, the MgO phase is structured further, which decreases in its specific surface area and basicity.
Similar content being viewed by others
REFERENCES
Ermakov, Yu.I., Zakharov, V.A., and Kuznetsov, B.N., Zakreplennye kompleksy na oksidnykh nositelyakh v katalize (Fixed Complexes on Oxide Carriers in Catalysis), Novosibirsk: Nauka, 1980.
Catalyst Supports and Supported Catalyst, Stiles, A.B., Ed., Boston: Butterworth, 1987.
Napolniteli dlya polimernykh materialov (Filling Materials for Polymer Composite Materials), Babaevskii, P.G., Ed., Moscow: Khimiya, 1981.
Petrova, L.I., Malkov, A.A., and Malygin, A.A., Zh. Prikl. Khim., 1991, vol. 64, no. 71435.
Adsorption on New and Modified Inorganic Sorbents, Dabrowski, A. and Tertykh, V.A., Eds., Amsterdam: Elsevier, 1996, vol. 99, p. 213.
Malygin, A.A., Zh. Prikl. Khim., 1996, vol. 69, no. 10, p. 1585.
Dzis'ko, V.A., Karnaukhov, A.P., and Tarasova, D.V., Fiziko-khimicheskie osnovy sinteza oksidnykh katalizatorov (Physicochemical Fundamentals of the Synthesis of Oxide Catalysts), Novosibirsk: Nauka, 1978.
Treushchenko, N.N., Dmitrievskii, B.A., Kebryakova, N.V., and Belitskaya, A.O., Zh. Prikl. Khim., 1994, vol. 67, no. 4, p. 550.
Choudhary, V.R., Rane, V.H., and Gadre, R.V., J. Catal., 1994, vol. 145, no. 2, p. 300.
Tret'yakov, Yu.D., Tverdofaznye reaktsii (Solid-Phase Reactions), Moscow: Khimiya, 1978.
Gusarov, V.V., Malkov, A.A., Malygin, A.A., and Suvorov, S.A., Zh. Prikl. Khim., 1994, vol. 67, no. 6, p. 935.
Gusarov, V.V., Malkov, A.A., Malygin, A.A., and Suvorov, S.A., Neorg. Mater., 1995, vol. 31, no. 3, p. 346.
Hamano, K. and Katafuchi, S., Taikabutsu, 1980, vol. 32, no. 3, p. 243.
Vaivad, A.Ya., Magnezial'nye vyazhushchie veshchestva (Magnesia Cements), Riga: Zinatne, 1971.
Zubakov, S.M., Babin, P.N., and Akishev, A.Kh., Ogneupory, 1979, no. 5, p. 47.
Hamano, K., Taikabutsu, 1985, vol. 37, no. 3, p. 124.
Tanabe, K., Solid Acids and Bases. Their Catalytic Properties, New York: Academic, 1970.
Nefedov, V.I., Rentgenoelektronnaya spektroskopiya khimicheskikh soedinenii (X-ray Electron Spectroscopy of Chemical Compounds: Handbook), Moscow: Khimiya, 1984.
Samoilov, V.M. and Ryabov, A.N., Kinet. Katal., 1978, vol. 19, no. 1, p. 250.
Nechiporenko, A.P. and Kudryashova, A.I., Zh. Prikl. Khim., 1987, vol. 60, no. 9, p. 1957.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Morozov, S.A., Malkov, A.A. & Malygin, A.A. Synthesis of Porous Magnesium Oxide by Thermal Decomposition of Basic Magnesium Carbonate. Russian Journal of General Chemistry 73, 37–42 (2003). https://doi.org/10.1023/A:1023466200445
Issue Date:
DOI: https://doi.org/10.1023/A:1023466200445