Abstract
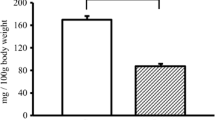
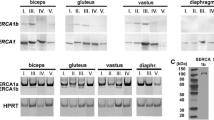
The question whether aquaporin 3 (AQP3) is expressed in normal human skeletal muscle at mRNA and protein levels has been examined, since AQP3 has been reported to be coexpressed with AQP4 in various kinds of tissues other than skeletal muscle. The gel electrophoresis of the reverse transcription polymerase chain reaction (RT-PCR) product of total RNA samples extracted from normal human muscle specimens by using the oligonucleotide primers for AQP3 contained a band of 629 base pairs which corresponded to the base pair length between two primers of AQP3. The nucleotide sequence of this RT-PCR product coincided with that of AQP3. At the protein level, immunoblot, immunohistochemical and immunoelectron microscopical studies were done by using rabbit antibody against the synthetic peptide of the cytoplasmic domain of the human AQP3 molecule. Immunoblot analysis showed that rabbit antibody against the human AQP3 reacted with a protein of approximately 30 kDa molecular weight in extracts of normal human skeletal muscles. The immunoreaction for the anti-AQP3 antibody with normal human muscle was noted at the myofibre surface. Immunogold labelling electron microscopy revealed that the gold particles indicating the presence of AQP3 molecules were located mainly at the inside surface of muscle plasma membrane.
Similar content being viewed by others
References
Denker BM, Smith BL, Kuhajda FP, Agre P (1988) Identification, purifi-cation, and partial characterization of a novelMr 28,000 integral membrane protein from erythrocytes and renal tubules. J Biol Chem 263: 15634–15642.
Echevarria M, Windhager EE, Tate SS, Frindt G (1994) Cloning and expression of AQP3, a water channel from the medullary collecting duct of rat kidney. Proc Natl Acad Sci USA 91: 10997–11001.
Frigeri A, Gropper MA, Turck CW, Verkman AS (1995) Immunolocalization of the mercurial-insensitivewater channel and glycerol intrinsic protein in epithelial cell plasma membranes. Proc Natl Acad Sci USA 92: 4328–4331.
Frigeri A, Nicchia GP, Nico B, Quondamatteo F, Herken R, Roncali L, Svelto M (2001) Aquaporin-4 deficiency in skeletal muscle and brain of dystrophic mdx mice. FASEB J 15: 90–98.
Frigeri A, Nicchia GP, Verbavatz JM, Valenti G, Svelto M (1998) Expression of aquaporin-4 in fast-twitch fibers of mammalian skeletal muscle. J Clin Invest 102: 695–703.
Hasegawa H, Ma T, Skach W, Matthay M, Verkman AS (1994) Molecular cloning of a mercurial-insensitive water channel expressed in selected water-transporting tissues. J Biol Chem 269: 5497–5500.
Inase N, Fushimi K, Ishibashi K, Uchida S, Ichioka M, Sasaki S, Marumo F (1995) Isolation of human aquaporin 3 gene. J Biol Chem 270: 17913–17916.
Ishibashi K, Sasaki S, Saito F, Ikeuchi T, Marumo F (1995) Structure and chromosomal localization of a humanwater channel (AQP3) gene. Genomics 27: 352–354.
Ishibashi K, Sasaki S, Fushimi K, Uchida S, Kuwahara M, Saito H, Furukawa T, Nakajima K, Yamaguchi Y, Gojobori T, Marumo F (1994) Molecular cloning and expression of a member of the aquaporin family with permeability to glycerol and urea in addition to water expressed at the basolateral membrane of kidney collecting duct cells. Proc Natl Acad Sci USA 91: 6269–6273.
Jimi T, Wakayama Y, Murahashi M, Shibuya S, Inoue M, Hara H, Matsuzaki Y, Uemura N (2000) Aquaporin 4: Lack of mRNA expression in the rat regenerating muscle fiber under denervation. Neurosci Lett 291: 93–96.
Liu JW, Wakayama Y, Inoue M, Shibuya S, Kojima H, Jimi T, Oniki H (1999) Immunocytochemical studies of aquaporin 4 in the skeletal muscle of mdx mouse. J Neurol Sci 164: 24–28.
Ma T, Frigeri A, Hasegawa H, Verkman AS (1994) Cloning of a water channel homolog expressed in brain meningeal cells and kidney collecting duct that functions as a stilbene-sensitive glycerol transporter. J Biol Chem 269: 21845–21849.
Preston GM, Agre P (1991) Isolation of thecDNAfor erythrocyte integral membrane protein of 28 kilodaltons: Member of an ancient channel family. Proc Natl Acad Sci USA 88: 11110–11114.
Preston GM, Carroll TP, Guggino WP, Agre P (1992) Appearance of water channels in Xenopus oocytes expressing red cell CHIP28 protein. Science 256: 385–387.
Umenishi F, Verkman AS, Gropper MA (1996) Quantitative analysis of aquaporin mRNA expression in rat tissues by RNase protection assay. DNA Cell Biol 15: 475–480.
Wakayama Y, Jimi T, Inoue M, Kojima H, Murahashi M, Kumagai T, Yamashita S, Hara H, Shibuya S (2002) Reduced aquaporin 4 expression in the muscle plasma membrane of patients with Duchenne muscular dystrophy. Arch Neurol 59: 431–437.
Wakayama Y, Jimi T, Takeda A, Misugi N, Kumagai T, Miyake S, Shibuya S (1990) Immunoreactivity of antibodies raised against synthetic peptide fragments predicted from mid portions of dystrophin cDNA. J Neurol Sci 97: 241–250.
Wakayama Y, Kojima H, Inoue M, Murahashi M, Shibuya S, Takahashi J, Oniki H (2001) Confocal laser and immunoelectron microscopic studies of aquaporin 4 localization in normal skeletal myofiber. Acta Myologica 20: 125–129.
Yang B, Verbavatz JM, Song Y, Vetrivel L, Manley G, Kao WM, Ma T, Verkman AS (2000) Skeletal muscle function and water permeability in aquaporin-4 deficient mice. Am J Physiol Cell Physiol 278: C1108–C1115.
Yokota T, Miyagoe Y, Hosaka Y, Tsukita K, Kameya S, Shibuya S, Matsuda R, Wakayama Y, Takeda S (2000) Aquaporin 4 is absent at the sarcolemma and at perivascular astrocyte endfeet in alpha1-syntrophin knockout mice. Proc Jpn Acad 76: 22–27.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Wakayama, Y., Jimi, T., Inoue, M. et al. Expression of Aquaporin 3 and its Localization in Normal Skeletal Myofibres. Histochem J 34, 331–337 (2002). https://doi.org/10.1023/A:1023382609541
Issue Date:
DOI: https://doi.org/10.1023/A:1023382609541