Abstract
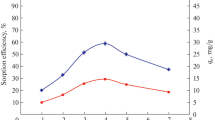
The uptake behavior of Sb(V) onto diphenylthiocarbazone (H2DZ) loaded polyurethane foam (PUF) from aqueous solutions of different acids in the presence of KI have been studied. The maximum adsorption was found from 0.5N HNO3 containing 0.2M KI. The maximum equilibrium was achieved within twenty minutes shaking time. The sorption behavior followed the Freundlich and Langmuir adsorption isotherms. The Freundlich constants 1/n and Kf are 0.57 and 3.26.10-2 mol.g-1, respectively. The Langmuir constants M and b are 2.18.10-4 mol.g-1 and 2.4.104 l.g-1, respectively. The value of sorption free energy (E) evaluated from D-R isotherm is 10.8 kJ.mol-1 indicating the ion exchange type chemisorption of Sb(V) on H2DZ loaded PUF. The thermodynamic parameters of enthalpy (ΔH), entropy (ΔS) and Gibbs free energy (ΔG) have also been investigated and found to be -51.8 kJ.mol-1, -127.3 J.mol-1.deg-1 and -13.8 kJ.mol-1, respectively. The negative values of (ΔH) and (ΔG) indicate that the sorption is exothermic and spontaneous in nature. The effect of anions and cations and sorption mechanism are discussed.
Similar content being viewed by others
References
S. PalÁgyi T. Braun, in: Preconcentration Techniques for Trace ElementsZ. B. Alfassi C. M. Wai (Eds), CRC Press, Boca Raton, 1992, p. 336.
M. M. Saeed A. Rusheed, Sci. Int., 10 (1998) 273.
A. Raychaudhuri S. K. Roy, Talanta, 41 (1994) 171.
I. Valente H. J. M. Bowen, Analyst, 102 (1977) 842.
S. M. Hasany M. M. Saeed M. Ahmed, Talanta, 54 (2001) 89.
M. M. Saeed A. Ghaffar, J. Radioanal. Nucl. Chem., 232 (1998) 271.
S. M. Hasany M. M. Saeed M. Ahmed, Separ. Sci. Technol., 35 (2000) 379.
S. M. Hasany M. M. Saeed M. Ahmed, Separ. Sci. Technol., 36 (2001) 555.
M. M. Saeed A. Rusheed N. Ahmed J. TÖlgyessy, Separ. Sci. Technol., 29 (1994) 2143.
M. M. Saeed A. Rusheed, Radiochim. Acta, 90 (2002) 35.
H. J. M. Bowen, J. Chem. Soc., A (1970) 1082.
M. M. Saeed S. M. Hasany M. Ahmed, Talanta, 50(1999) 625.
H. Freundlich, Colloid and Capillary Chemistry, Methuen, London, 1926, p. 397.
I. Langmuir, J. Am. Chem. Soc., 40 (1918) 1361.
M. M. Dubinin L. V. Radushkevich, Proc. Acad. Sci., USSR, Phys. Chem. Soc., 55 (1947) 331.
F. A. Cotton G. Wilkinson C. A. Murillo M. Bochmann, Advanced Inorganic Chemistry, 6th ed., John Wiley & Sons, Inc., 1999, p. 396.
H. Remy, Treatise on Inorganic Chemistry, Elsevier, London, 1956, p. 672.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Saeed, M.M., Ahmed, M. & Ghaffar, A. Adsorption modeling of antimony(V) on diphenylthiocarbazone loaded polyurethane foam. Journal of Radioanalytical and Nuclear Chemistry 256, 121–126 (2003). https://doi.org/10.1023/A:1023364428987
Issue Date:
DOI: https://doi.org/10.1023/A:1023364428987