Abstract
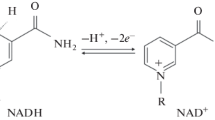
The kinetics of reduction of ferricytochrome c in its reaction with thiamine and its O-substituted structural analogs in phosphate buffer at pH 7.5-7.8 were studied. The reduction rate is proportional to the concentration of thiamine or its derivatives. The dependence of the reaction rate on the oxidant concentration is characterized by negative deviations from linearity. In oxidation with ferricytochrome c, the reactivity of thiamine consdierably exceeds the reactivity of thiamine diphosphate and thiamine monophosphate, and in oxidation with ferricyanide the reaction rate increases in the order thiamine monophosphate < thiamine < thiamine diphosphate. With O-(2-norbornoyl)thiamine, O-(2-adamantyl)acetylthiamine, O-benzoylthiamine, O-(4-nitrobenzoyl)thiamine, or O-(5-nitro-2-chlorobenzoyl)thiamine, the rate of ferricytochrome c reduction is higher than with thiamine. Presumably, the electron transfer to the heme group of the oxidant is preceded by formation of a complex of ferricytochrome c with the neutral tricyclic form of the substrate.
Similar content being viewed by others
REFERENCES
Risinger, G.E. and Parker, P.N., Experientia, 1965, vol. 21, no. 6, p. 305.
Penttinen, H.K., Acta Chem. Scand. (B), 1976, vol. 30, no. 7, p. 659.
Stepuro, I.I., Piletskaya, T.P., Stepuro, V.I., and Maskevich, S.A., Biokhimiya, 1997, vol. 62, no. 12, p. 1648.
Melnichenko, N.G., Zverinsky, I.V., Artsukevich, I.M., and Zukienko, P.I., Exp. Toxicol. Pathol. (B), 1999, vol. 51, no. 4, p. 389.
Li, G., Cook, M.E., and Wu, W., Biochem. Biophys. Res. Commun., 1996, vol. 226, no. 1, p. 187.
Pique, M., Barragan, M., Dalmau, M., Bellosillo, B., Pons, G., and Gil, J., FEBS Lett., 2000, vol. 480, nos. 2–3, p. 193.
Yamada, T., Kikawa, K., Shinoda, S., and Tsukube, H., Tetrahedron Lett., 1999, vol. 40, no. 38, p. 6967.
Avigliano, L., Carelli, V., Casini, A., Finazzi-Agro, A., Liberatore, F., and Rossi, A., Biochem. J., 1986, vol. 237, p. 919.
Vovk, A.I., Murav'eva, I.V., Kukhar', V.P., and Baklan, V.F., Zh. Obshch. Khim., 2000, vol. 70, no. 7, p. 1181.
Herrmann, J., Knoche, W., and Heugebauer, R., J. Chem. Soc., Perkin Trans. 2, 1995, no. 3, p. 463.
Kozik, A., Thiamine—Protein Interaction, Krakow, 1996, p. 133.
Rapala-Kozik, M. and Kozik, A., Biochim. Biophys. Acta, 1992, vol. 1159, no. 2, p. 209.
Postoenko, V.V., Parkhomenko, Yu.M., Vovk, A.I., Khalmuradov, A.G., and Donchenko, G.V., Biokhimiya, 1987, vol. 52, no. 11, p. 1792.
Matsukawa, T., Hirano, H., and Yurugi, S., Meth. Enzymol. (A), 1970, vol. 18, p. 141.
Vovk, A.I., Murav'eva, I.V., and Parkhomenko, Yu.M., Ukr. Biokhim. Zh., 2000, vol. 72, no. 3, p. 124.
Stivers, J.T. and Waschabaugh, M.W., Bioorg. Chem., 1992, vol. 20, no. 2, p. 155.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Vovk, A.I., Babii, L.V. & Murav'eva, I.V. Reduction of Cytochrome c in Its Reaction with Thiamine and Its Structural Analogs. Russian Journal of General Chemistry 72, 1808–1812 (2002). https://doi.org/10.1023/A:1023361817590
Issue Date:
DOI: https://doi.org/10.1023/A:1023361817590