Abstract
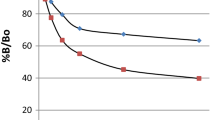
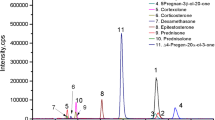
Purification of the radioiodinated progesterone tyrosine methyl ester conjugate by gel filtration and the development, optimization and clinical validation of a direct radioimmunoassay (RIA) of progesterone using this radiotracer are described. High purity radiotracer is essential for the error free performance of any RIA. Progesterone 11α hemisuccinate was conjugated to tyrosine methyl ester (TME) by the mixed anhydride method and this conjugate was then radioiodinated by the chloramine-T method. Purification of the radioiodinated product was carried out by gel filtration. About 12 batches of the radiotracer were prepared and purified. The purification by gel filtration gave reproducible elution pattern and purity. The radiotracer thus purified was found to have consistent quality as compared to that of any other purification methods. Non-specific binding of the radiotracer was found to be <5% in second antibody-polyethylene glycol based separation. 'Zero standard' binding was found to be consistently 50-60% in all the batches of radiotracer. The radiochemical purity of all the batches of radiotracer was >95% as checked by paper electrophoresis. The stability (retention of the immunoreactivity) of the radiotracer was two to three months. No appreciable changes in the assay characteristics were observed during this period. The assay involved 3 hours incubation of progesterone antibody with individual standards or sample and radiotracer at room temperature. The optimized assay was then validated for internal and external quality control parameters. A RIA kit was then formulated with this radiotracer for estimation of progesterone in serum. The assay kit consisted of lyophilized individual standards ranging from 0.25 to 50 ng/ml. The clinical performance of the developed kit was compared with that of a commercial ELISA kit and a correlation of 0.94 was observed.
Similar content being viewed by others
References
J. Zander T. R. Forbes A. M. von Munstermann R. Neher, J. Clin. Endocrinol. Metab., 18 (1958) 337.
E. Diczfalusy, Acta Endocrinol., 10 (1952) 373.
A. M. Moses J. Lobotsky C. W. Lloyd, J. Clin. Endocrinol. Metab., 19 (1959) 987.
E. D. B. Johansson, Acta Endocrinol., 61 (1969) 592.
B. S. Lindberg B. A. Nilsson E. D. B. Johansson, Acta Obstet. Gynec. Scand., 53 (1974) 329.
E. H. D. Cameron J. J. Scarisbrick, Clin. Chem., 19 (1973) 1403.
K. K. Dighe W. M. Hunter, Biochem. J., 143 (1974) 219.
M. Kutas A. Chung D. Bartos A. Castro, Steroids, 20 (1972) 697.
I. H. Thorney Croft S. C. Stone, Conception, 5 (1972) 129.
D. C. Anderson, Clin. Chim. Acta, 29 (1970) 513.
G. E. Abrahman, in: Modern Methods in Steroid AnalysisHeftmann E. (Ed.), Academic Press, New York, 1973, p. 451.
R. P. Ekins, Radioimmunoassay and Related Procedures in Medicine, IAEA, Vienna, 1978, p. 6.
K. Kothari M. R. A. Pillai, J. Radioanal. Nucl. Chem., 177 (1994) 261.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Karir, T., Pal, N. & Sivaprasad, N. Radioimmunoassay kit formulation and its validation for serum progesterone using progesterone radiotracer purified by gel filtration. Journal of Radioanalytical and Nuclear Chemistry 256, 127–131 (2003). https://doi.org/10.1023/A:1023316513057
Issue Date:
DOI: https://doi.org/10.1023/A:1023316513057