Abstract
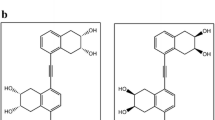
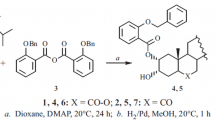
A new synthetic brassinolide analogue, 2α,3α-dihydroxy-17β-(3-methylbutyryloxy)-7-oxa-B-homo-5α-androstan-6-one (11), has been shown to exhibit typical brassinolide activity characterised by elongation, swelling, twisting and splitting of the bean second internode. It was prepared from the known lactone 2α,3α,17β-trihydroxy-7-oxa-B-homo-5α-androstan-6-one (4) which was transformed to an isopropylidenedioxy derivative. After protection of the 2α- and 3α-hydroxy groups it yielded the 2α,3α-isopropylidenedioxy-17β-(3-methyl-butyryloxy)-7-oxa-B-homo-5α-androstan-6-one (7) on treating with 3-methylbutyryl chloride in pyridine. The analogue with a 2-methylbutyric moiety (10, 2α,3α-dihydroxy-17β-(2-methyl-butyryloxy)-7-oxa-B-homo-5α-androstan-6-one) in position 17β stimulated only elongation and swelling of the bean second internode. However, in this bioassay 100 times more 10 or 11 compared to 24-epibrassinolide is required to obtain the same effects. Analogues with β-oriented hydroxyl groups at C-2 and C-3 (14,15), a 6-ketone (17,18) or 6-oxa-7-oxo-lactone system (12,13) in ring B lack the typical brassinolide activity. In addition, the active brassinosteroids applied to the second internode stimulated a similar, but 30% lower elongation of the first internode. From data presented here we conclude that the presence of two hydroxy groups in the positions 22 and 23 of the brassinolide side chain, which are considered as a key structural requirement, is not absolutely necessary for a compound to exhibit typical brassinosteroid activity. Nevertheless, these compounds have generally 2–10 times lower activity than that having 22,23-vicinal diol in the side chain.
Similar content being viewed by others
Rerences
Barlow P. 1987. Requirements for hormone involvement in development at different levels of organization. In: Hormone Action in Plant Development. Butterworths, London, pp. 39–52.
Černý V., Strnad M. and Kamínek M. 1986. Preparation of 2α,3adihydroxy-7-oxa-6-oxo-23,24-dinor-B-homo-5α-cholanic acid, its esters and amides as brassinolide analogues. Coll. Czech. Chem. Commun. 51: 687–697.
Černý V., Zajíček J. and Strnad M. 1987. 2α,3α-dihydroxy-7-oxa-6-oxo-B-homo-5α-pregnan-21-oic acid and its derivatives as brassinolide analogues. Coll. Czech. Chem. Commun. 52: 215–222.
Fung S. and Siddall J.B. 1980. Stereoselective synthesis of brassinolide: A plant growth promoting steroidal lactone. J. Am. Chem. Soc. 102: 6580–6581.
Gregory L.E. and Mandava N.B. 1982. The activity and interaction of brassinolide and gibberellic acid in mung bean (Phaseolus aureus L.) epicotyls. Physiol. Plant. 54: 239–243.
Grove M.D., Spencer G.F., Rohwedder W.K., Mandava N.B., Worley J.F., Warthen J.D. et al. 1979. Brassinolide, a plant growthpromoting steroid form Brassica napus pollen. Nature 281: 216–217.
Ishiguro M., Takatsuto S., Morisaki M. and Ikekawa N. 1980. Synthesis of brassinolide, a steroidal lactone with plant-growth promoting activity. J. Chem. Soc., Chem. Commun. 20: 962–964.
Kishi T., Wada K., Maruma S. and Mori K. 1986. Synthesis of brassinolide analogs with a modified ring B and their plant-promoting activity. Agric. Biol. Chem. 5: 1821–1830.
Kohout L. and Strnad M. 1986. Brassinosteroids with a cholestane side chain. Coll. Czech. Chem. Commun. 51: 447–458.
Kohout L., Velgová H., Strnad M. and Kamínek M. 1987. Brassinosteroids with androstane and pregnane skeleton. Coll. Czech. Chem. Commun. 52: 476–486.
Kohout L., Černý V. and Strnad M. 1987. Alternative synthesis of 2α,3α,17β-trihydroxy-7-oxa-B-homo-5α-androstan-6-one and some androstane brassinolide analogs. Coll. Czech. Chem. Commun. 52: 1026–1042.
Kohout L. 1989. Synthesis of brassinosteroids with a five carbon atom ester functionality in position 17. Coll. Czech. Chem. Commun. 54: 3348–3359.
Kohout L., Strnad M. and Kamínek M. 1991. Types of brassinosteroids and their bioassays. In: Cutler H.G., Yokota T. and Adam G. (eds), Brassinosteroids: Chemistry, Bioactivity, and Applications., Washington, pp. 56–73.
Mandava N.B. 1988. Plant growth-promoting brassinosteroids. Annu. Rev. Plant Physiol. Plant. Mol. Biol. 39: 23–52.
Mitchell J.W. and Livingstone G.A. 1968. Methods of Studying Plant Hormones and Growth Regulating Substances. Agriculture Handbook No. 336. U.S. Government Printing Office, Washington, D.C.
Mitchel J.W., Mandava N.B., Worley J.F., Plimmer J.R. and Smith M.V. 1970. Brassin-a new family of plant hormones from rape pollen. Nature 225: 1065–1066.
Sakakibara M., Okada K., Ichikawa Z. and Mori K. 1982. Synthesis of brassinolide, a plant growth promoting steroidal lactone. Heterocycles 17: 301–304.
Sasse J.M. 1985. The place of brassinolide in the sequential response to plant growth regulators in elongating tissue. Physiol. Plant. 63: 303–308.
Sasse J.M. 1991. Brassinolide-induced elongation. In: Cutler H.G., Yokota T. and Adam G. (eds), Brassinosteroids: Chemistry, Bioactivity, and Applications., Washington, pp. 255–264.
Strnad M., Motyka V. and Kamínek M. 1985. Different activities of four brassinosteroids in sensitized bean first and second internode bioassay. In: Heidelberg: 12th Inter Conf Plant Growth Subst, Abstr No. 0605.
Takatsuto S., Yazawa H., Ikekawa N., Takematsu T., Takeuchi Y. and Koguchi M. 1983. Structure-activity relationship of brassinosteroids. Phytochemistry 22: 2437–2441.
Takatsuto S. and Ikekawa N. 1984. Stereoselective synthesis of plant growth-promoting steroids, brassinolide, castasterone, typhasterol and their 28-nor analogues. J. Chem. Soc. Perkin Trans. 1: 139–146.
Takatsuto S., Yazawa N. and Ikekawa N. 1984. Synthesis and biological activity of brassinolide analogues, 26,27-bisnorbrassinolide and its 6-oxo analogue. Phytochemistry 23: 525–528.
Thompson M.J., Mandava N.B., Fllippen-Anderson J.L., Worley J.F., Dutky S.R., Robbins W.E. et al. 1979. Synthesis of brassinosteroids. New plant growth promoting steroids. J. Org. Chem. 44: 5002–5004.
Thompson M.J., Mandava N.B., Meudt W.J., Lusby W.R. and Spaulding D.W. 1981. Synthesis and biological activity of brassinolide and its 22β-23β-isomer: novel plant growth promoting steroids. Steroids 38: 567–580.
Thompson M.J., Meudt W.J., Mandawa N.B., Dutky S.R., Lusby W.R. and Spaulding D.W. 1982. Synthesis of brassinosteroids and relationship of structure to plant growth-promoting steroids. Steroids 39: 89–105.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Strnad, M., Kohout, L. A simple brassinolide analogue 2α, 3α-dihydroxy-17β-(3-methyl-butyryloxy)-7-oxa-B-homo-5α-androstan-6-one which induces bean second internode splitting. Plant Growth Regulation 40, 39–47 (2003). https://doi.org/10.1023/A:1023091918892
Issue Date:
DOI: https://doi.org/10.1023/A:1023091918892