Abstract
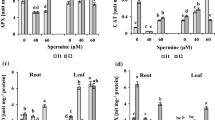
The effects of putrescine and ethephon on peroxidase (POD; EC 1.11.1.7), polyphenol oxidase (PPO; EC 1.14.18.1), catalase (CAT; EC 1.11.1.6) activities and proline content in spinach leaves under saline stress were investigated. In control conditions, putrescine increased PPO and CAT activities and proline content, but decreased POD activity. Ethephon increased these three enzyme activities but did not affect proline content. In saline conditions, putrescine increased POD and CAT activities and proline content, while it decreased PPO activity. Ethephon increased both PPO and CAT activities and proline content, but decreased POD activity. Putrescine and ethephon have opposite effects on the enzyme activities and proline accumulation because they acts as antagonists.
Similar content being viewed by others
References
Abeles F.B., Dunn L.J., Morgens P., Callahan A., Dinterman R.E. and Schmidt J. 1988. Induction of 33-kD and 60-kD peroxidases during ethylene-induced senescence of cucumber cotyledons. Plant Physiol. 87: 609–615.
Angelini R., Manes F. and Federico R. 1990. Spatial and functional correlation between diamine-oxidase and peroxidase activities and their dependence upon de-etiolation and wounding in chick-pea stems. Planta 182: 89–96.
Anthony B.B. and Schaller G.E. 1996. The mechanism of ethylene perception. Plant Physiol. 111: 653–660.
Apelbaum A., Alan C., Burgoon James D. and Anderson L. 1981. Polyamines inhibit biosynthesis of ethylene in higher plant tissue and fruit protoplasts. Plant Physiol. 68: 453–456.
Arslan O., Temur A. and Tozlu I. 1997. Polyphenol oxidase from Allium sp. J. Agric. Food Chem. 45: 2861–2863.
Atienzar J., Angeles M. and Garcia-Carmona F. 1991. Activation of polyphenol oxidase by polyamines. Biochem. Int. Dec. 25: 861–868.
Aziz A., Martin-Tanguy J. and Larher F. 1999. Salt stress-induced proline accumulation and changes in tyramine and polyamine levels are linked to ionic adjustment in tomato leaf discs. Plant Sci. 145: 83–91.
Bagni N. and Serafini-Fracassini D. 1985. Involvement of polyamines in the mechanism of break of dormancy in Helianthus tuberosus. Bull. Soc. Bot. Fr. (Actual. Bot.) 132: 119–125.
Bagni N., Bongiovanni B., Franceshetti M. and Tassoni A. 1998. Abscisic acid mutants affect polyamine metabolism. Plant Biosyst. 131: 181–187.
Bates L.S., Waldren R.P. and Teare I.D. 1973. Rapid determination of free proline for water-stress studies. Plant Soil 39: 205–207.
Bouchereau A., Aziz A., Larher F. and Martin-Tanguy J. 1999. Polyamines and environmental challenges: recent development. Plant Sci. 140: 103–125.
Cassab G.I., Lin J.J., Lin L.S. and Varner J.E. 1988. Ethylene effect on extension and peroxidase distribution in the subapical region of pea epicotyls. Plant Physiol. 88: 522–524.
Couture R., Cantwell M.I., Ke D.M.E. and Saltveith M.E. Jr. 1993. Physiological attributes related to quality attributes and storage life of minimally processed lettuce. Hort. Sci. 28: 723–725.
Demir Y. 2000. Growth and proline content of germinating wheat genotypes under ultraviolet light. Turk J. Bot. 24: 67–70.
Flurkey W.H. 1986. Polyphenol oxidase in higher plants. Plant Physiol. 81: 614–618.
Gahagan H.E., Holm R.E. and Abeles F.B. 1968. Effect of ethylene on peroxidase activity. Physiol. Plant 21: 1270–1279.
Hanson A.D. and Hitz W.D. 1982. Metabolic responses of Mesophytes to plant water deficits. Ann. Rev. Plant Physiol. 33: 163–203.
Hoagland D.R. and Arnon D.I. 1982. The water culture methods for growing plant without soil. Calif AES Circ. 347: 1–39.
Ke D. and Saltveith M.E. Jr. 1988. Plant hormone interaction and phenolic metabolism in the regulation of russet spotting in iceberg lettuce. Plant Physiol. 88: 1136–1140.
Kocaçalişkan I. and Kabar K. 1990. Effect of salinity on polyphenol oxidase during seed germination. Dogă-Tr. J. of Botany 15: 41–49.
Kushad M.M. and Dumbroff E.B. 1991. Metabolic and physiological relationship between the polyamine and ethylene biosynthetic pathways. In: Slocum R.D. and Flores H.E. (eds), The Biochemistry and Physiology of Polyamines in Plants. CRC Press, Boca Raton, FL, pp. 78–89.
Lück H. 1965. Catalase. In: Bergmeyer H.U. (ed.), Methods of Enzymatic Analysis. Academic Press, New York, pp. 885–894.
Morgan P.W. and Drew M.C. 1997. Ethylene and plant response to salt stress. Physiol. Plant 100: 620–630.
Pandey R.M. and Ganaphaty P.S. 1985. The proline enigma: NaCI-tolerant and NaCI-sensitive callus lines of Cicer arietinum. Plant Sci. 40: 13–17.
Partington J.C. and Bolwell P.G. 1996. Purification of polyphenol oxidase free of the storage protein patatin from potato tuber. Phytochemistry 42: 1499–1502.
Peng X.X. and Yamauchi M. 1993. Ethylene production in rice bronzing leaves induced by ferrous iron. Plant Soil 149: 227–234.
Retig N. and Rudich J. 1972. Peroxidase and IAA oxidase activity and isoenzyme patterns in cucumber plants, as affected by sex expression and ethephon. Physiol. Plant 27: 156–160.
Sancho M.A., Milrad de Forchetii S., Pliego F., Valpuesta V. and Queseda M.A. 1996. Total peroxidase activity and isoenzymes in the culture medium of NaCI adapted tomato suspension cells. Plant Cell Tiss. Org. Cult. 44: 161–167.
Siegel B. 1993. Plant peroxidases an organismic perspective. Plant Growth Reg. 12: 303–312.
Smith M.A., Davies R.J. and Reid J.B. 1985. Role of polyamines in gibberellin-induced internode growth in pea. Plant Physiol. 78: 92–99.
Sreenivasulu N., Ramanjulu S., Ramachandra-Kini K., Prakash H.S., Shekar-Shetty H., Savithri H.S. et al. 1999. Total peroxidase activity and peroxidase isoforms as modified by salt stress in two cultivars of fox-tail millet with differential salt tolerance. Plant Sci. 141: 1–9.
Takemura T., Hanagata N., Sugihara K., Baba S., Karube I. and Dubinsky Z. 2000. Physiological and biochemical responses to salt stress in the mangrove, Bruguiera gymnorrhiza. Aquat. Bot. 68: 15–28.
Tiburcio A.F., Campos J.L., Figuoras X. and Besford R.T. 1993. Recent advances in the understanding of polyamines functions during plant development. Plant Growth Regul. 12: 331–340.
Trotel P., Bouchereau A., Niogret M.F. and Larher F. 1989. The fate of osmo-accumulated proline in leaf discs of rape (Brassica napus L.) incubated in a medium of low osmolarity. Plant Sci. 118: 31–45.
Velikova V., Yordanov I. and Edreva A. 2000. Oxidative stress and some antioxidant systems in acid rain-treated bean plants. Protective role of exogenous polyamines. Plant Sci. 151: 59–66.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Öztürk, L., Demir, Y. Effects of putrescine and ethephon on some oxidative stress enzyme activities and proline content in salt stressed spinach leaves. Plant Growth Regulation 40, 89–95 (2003). https://doi.org/10.1023/A:1023078819935
Issue Date:
DOI: https://doi.org/10.1023/A:1023078819935