Abstract
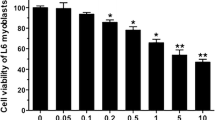
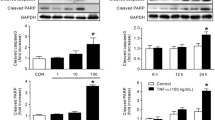
Treatment of C2C12 myotubes with a tumour-derived proteolysis-inducing factor (PIF) at concentrations between 1 and 10 nM was shown to stimulate the activity of the apoptotic initiator caspases-8 and -9 and the apoptotic effector caspases-2, -3 and -6. This increased caspase activity was attenuated in myotubes pretreated with 50 μM eicosapentaenoic acid (EPA). At least part of the increase in caspase activity may be related to the increased proteasome proteolytic activity, since a caspase-3 inhibitor completely attenuated the PIF-induced increase in ‘chymotrypsin-like’ enzyme activity, the predominant proteolytic activity of the proteasome. However, Western blot analysis showed that PIF induced an increase in expression of the active form of caspase-3, which was also attenuated by EPA.
Further Western blot analysis showed PIF increased the cytosolic content of cytochrome c, as well as expression of the pro-apoptotic protein bax but not the anti-apoptotic protein bcl-2, which were both attenuated by 50 μM EPA. Induction of apoptosis by PIF in murine myotubes was confirmed by an increase in free nucleasomes formation and increased DNA fragmentation evidenced by a nucleasomal ladder typical of apoptotic cells. This process was again inhibited by pre-incubation with EPA. These results suggest that in addition to activating the proteasome, PIF induces apoptosis in C2C12 myotubes, possibly through the common intermediate arachidonic acid. Both of these processes would contribute to the loss of skeletal muscle in cancer cachexia.
Similar content being viewed by others
References
Fearon KCH. The mechanisms and treatment of weight loss in cancer. Proc Nutri Soc 1992; 51: 251–265.
Bistrian BR, Blackburn GL, Scrimshaw NS, Flatt, JP. Cellular immunity in semistarved states in hospitalized adults. Am J Clin Nutr 1978; 28: 1148–1153.
Andreyev HJN, Norman AR, Oates J, Cunningham D. Why do patients with weight loss have a worse outcome when undergoing chemotherapy for gastrointestinal malignancies? Eur J Cancer 1998; 35: 503–509.
Todorov P, Cariuk P, McDevitt T, Coles B, Fearon K, Tisdale M. Characterization of a cancer cachectic factor. Nature 1996; 379: 739–742.
Smith HJ, Lorite MJ, Tisdale MJ. Effect of a cancer cachectic factor on protein synthesis/degradation in murine C2C12 myoblasts: Modulation by eicosapentaenoic acid. Cancer Res 1999; 59: 5507–5513.
Lorite MJ, Cariuk P, Tisdale MJ. Induction of muscle protein degradation by a tumour factor. Br J Cancer 1997; 76: 1035–1040.
Williams A, Sun X, Fischer JE, Hasselgren P-O. The expression of genes in the ubiquitin-proteasome proteolytic pathway is increased in skeletal muscle from patients with cancer. Surgery 1999; 126: 744–750.
Lorite MJ, Smith HJ, Arnold JA, Morris JA, Thompson MG, Tisdale MJ. Activation of ATP-ubiquitin-dependent proteolysis in skeletal muscle in vivo and murine myoblasts in vitro by a proteolysis-inducing factor (PIF). Br J Cancer 2001; 85: 297–302.
Allen DL, Linderman JK, Roy RR, et al. Apoptosis: A mechanism contributing to the remodelling of skeletal muscle in response to hindlimb unweighting. Am J Physiol 1997; 273: C579–C587.
Tews DS, Goebel HH, Meinck HM. DNA fragmentation and apoptosis-related proteins of muscle cells in motor neuron disorders. Acta Neurol Scand 1997; 96: 380–386.
van Royen M, Carbo N, Busquets S, et al. DNA fragmentation occurs in skeletal muscle during tumor growth: A link with cancer cachexia? Biochem Biophys Res Commun 2000; 270: 533–537.
Yoshida H, Ishiko O, Sumi T, Honda K, Hirai K, Ogita S. Expression of apoptosis regulatory proteins in the skeletal muscle of tumor-bearing rabbits. Jpn J Cancer Res 2001; 92: 631–637.
Belizario JE, Lorite MJ, Tisdale MJ. Cleavage of caspases-1,-3,-6,-8 and-9 substrates by proteases in skeletal muscle from mice undergoing cancer cachexia. Br J Cancer 2001; 84: 1135–1140.
Dimmeler S, Breitschopf K, Haendeler J, Zeiher AM. Dephosphorylation targets Bcl-2 for ubiquitin-dependent degradation: A link between the apoptosome and the proteasome pathway. J Exp Med 1999; 189: 1815–1822.
Todorov PT, McDevitt TM, Cariuk P, Coles B, Deacon M, Tisdale MJ. Induction of muscle protein degradation and weight loss by a tumor product. Cancer Res 1996; 56: 1256–1261.
Orino E, Tanaka K, Tamura T, Sone S, Ogura T, Ichihara A. ATP-dependent reversible association of proteasomes with multiple protein compartments to form 26S complexes that degrade ubiquitinated proteins in human HL-60 cells. FEBS Lett 1991; 284: 206–210.
Kisselev AF, Akopian TN, Castillo V, Goldberg AL. Proteasome active sites allosterically regulate each other, suggesting a cyclical bite-chew mechanism for protein breakdown. Molecular Cell 1999; 4: 395–402.
Whitehouse AS, Smith HJ, Drake JL, Tisdale MJ. Mechanism of attenuation of skeletal muscle protein catabolism in cancer cachexia by eicosapentaenoic acid. Cancer Res 2001; 61: 3604–3609.
Bossy-Wetzel E, Green DR. Caspases induce cytochrome c release from mitochondria by activating cytosolic factors. J Biol Chem 1999; 274: 17484–17490.
Attaix D, Taillandier D. The critical role of the ubiquitinproteasome pathway in muscle wasting in comparison to lysosomal and Ca2+-dependent systems. Adv Mol Cell Biol 1998; 27: 235–266.
Barber MD, Ross JA, Voss AC, Tisdale MJ, Fearon KCH. The effect of an oral nutritional supplement enriched with fish oil on weight-loss in patients with pancreatic cancer. Br J Cancer 1999; 81: 80–86.
Kidd VJ, Lahti JM, Teitz T. Proteolytic regulation of apoptosis. Cell Dev Biol 2000; 11: 191–201.
Cao Y, Pearman A, Zimmerman GA, McIntyre TM, Prescott SM. Intracellular unesterified arachidonic acid signals apoptosis. Proc Natl Acad Sci USA 2000; 97: 11280–11285.
Narula J, Pandey P, Arbustini EB, et al. Apoptosis in heart failure: Release of cytochrome c from mitochondria and activation of caspase-3 in human cardiomyopathy. Proc Natl Acad Sci USA 1999; 96: 8144–8149.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Smith, H.J., Tisdale, M.J. Induction of apoptosis by a cachectic-factor in murine myotubes and inhibition by eicosapentaenoic acid. Apoptosis 8, 161–169 (2003). https://doi.org/10.1023/A:1022970609579
Issue Date:
DOI: https://doi.org/10.1023/A:1022970609579