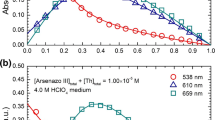
Abstract
A potentiometric study of the hydrolysis of arsenious acid was carried out to define the thermodynamic model of the inorganic arsenic species in aqueous solutions. The protonation equilibrium of arsenious acid was determined at 25°C. The variation of the stoichiometric formation constant with the ionic strength was also studied up to ionic strength 3.0 mol-dm−3 in aqueous NaClO4, NaCl, and KCl. The thermodynamic formation constant of arsenious acid (log K o = 9.22 ± 0.01) and the various interaction parameters were computed using the Modified Bromley Methodology (MBM), for both the molar and molal concentration scales at constant temperature (25°C). The results showed the importance, not only of ionic strength, but also of the composition of the ionic medium on the distribution of the acid-base As(III) species as a function of pH in natural waters.
Similar content being viewed by others
References
J. C. Raposo, Thesis, EHU, Leioa, Spain, 2001.
E. A. Woolson and N. Aharonson, J. Assoc. Off. Anal. Chem. 63, 523(1980).
R. L. Hothem and D. Welsh, Arch. Environ. Contam. Toxicol. 27, 180(1994).
M. E. Farago, Arsenic in the Marine Environment (Water Science and Technology Library, Palermo, Italy, 1997)
W. L. Lindsay and M. Sadiq, Sci. Total Environ. 28, 169(1983).
A. Gianguzza, E. Pelizzeti, and S. Sammartano, Marine Chemistry and Environmental Analytical Chemistry Approach (Kluwer Academic, Netherlands, 1997)
F. J. Millero and D. R. Schreiber, Amer. J. Sci. 282, 1508(1982).
T. M. Loehr and R. A. Plane, Inorg. Chem. 7, 1708(1972).
G. Borge, R. Castaño, M. P. Carril, M. S. Corbillón, and J. M. Madariaga, Fluid Phase Equilibr. 121, 85(1996).
G. Borge, N. Etxebarria, L. A. Fernandez, M. A. Olazabal, and J.M. Madariaga, Fluid Phase Equilibr. 121, 99(1996).
N. Etxebarria, L. A. Fernandez, and J.M. Madariaga, J. Chem. Soc. Dalton Trans., p. 3055(1994).
R. Castaño, N. Etxebarria, L. A. Fernandez, and J.M. Madariaga, J. Chem. Soc. Dalton Trans., p. 2729(1994).
G. Arana, N. Etxebarria, L. A. Fernandez, and J. M. Madariaga, J. Solution Chem. 24, 661(1995).
J. Sanz, J. C. Raposo, and J.M. Madariaga, Appl. Organomet. Chem. 14, 499(2000).
J. C. Raposo, J. Sanz, G. Borge, M. A. Olazabal, and J.M. Madariaga, Fluid Phase Equilibr. 155, 1(1999).
E. Bishop, Indicators (Pergamon Press, Germany, 1972)
G. H. Jeffery, J. Basset, J. Mendham, and R. C. Denney, Vogel's Textbook of Quantitative Chemical Analysis 5th edn. (Longman, London, UK, 1989)
R. Cazallas, L. A. Fernandez, N. Etxebarria, and J.M. Madariaga, Lab. Rob. Autom. 5, 161(1993).
G. Gran, Analyst 77, 661(1952).
F. J. C. Rossotti and H. Rossotti, The Determination of the Stability Constants (McGraw-Hill, New York, 1961)
J. L. Fonseca, Thesis, EHU, Leioa, Spain, 1989.
N. Ingri and L. G. Sillén, Acta Chem. Scand. 16, 173(1962).
Excel 97 (Microsoft Corporation, Redmond, WA, 1998)
C. de Stefano, P. Mineo, C. Rigano, and S. Sammartano, Ann. Chim. (Rome) 83, 243(1993).
C. de Stefano, C. Foti, O. Giuffré, P. Mineo, C. Rigano, and S. Sammartano, Ann. Chim. 86, 257(1996).
P. H. Sherrod, NLREG-Nonlinear Regression Analysis Program (Nashville, TN, 1995)
O. Sh÷nel and P. Novotny, Densities of Aqueous Solutions of Inorganic Substances (Czechoslovak Academy of Sciences, Praga, 1985)
A. Okumura and Y. Daido, Inorg. Chim. Acta 144, 63(1988).
L. G. Sillen and A. E. Martell, Stability Constants of Metal-Ion Complexes (Metcalfe & Cooper Limited, London, 1964)
C. F. Baes and R. E. Mesmer, The Hydrolysis of Cations (Wiley, New York, 1976)
J.C. Raposo, J. Sanz, O. Zuloaga, M.A. Olazabal, and J.M. Madariaga, Talanta 57, 849(2002).
J.C. Raposo, O. Zuloaga, M.A. Olazabal, and J.M. Madariaga, Fluid Phase Equilibr. in press.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Raposo, J.C., Sanz, J., Zuloaga, O. et al. Thermodynamic Model of Inorganic Arsenic Species in Aqueous Solutions. Potentiometric Study of the Hydrolytic Equilibrium of Arsenious Acid. Journal of Solution Chemistry 32, 253–264 (2003). https://doi.org/10.1023/A:1022938418459
Issue Date:
DOI: https://doi.org/10.1023/A:1022938418459