Abstract
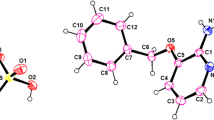
Upon evaporation at room temperature of an aqueous mixture containing Al(III) sulfate and trishydroxymethyl-ammoniummethane sulfate in a molar ratio 1:2, double sulfate as crystalline product was obtained. The stoichiometry of the obtained compound was determined by means of elemental and TG analysis. For identification, IR-spectra and X-ray powder diffraction patterns were done. It was found that the general formula of the obtained compound is Al(HOCH2)3CNH3(SO4)2·6H2O. as revealed by TG, DTG and DTA analysis, the dehydration of the AL-compound takes place in one step which points out that the six water molecules are bonded in the same way. The thermal decomposition of the anhydrous compound starts at about 260°C and is very complex. This process takes place in many steps which are not well resolved. The pathway of the thermal decomposition is also supposed.
Similar content being viewed by others
References
B. H. Serezhkin, 10 (1984) 20.
S. Haussuhl, Kristallography der Alaune, Z. Kristallogr. Mineral., 116 (1961) 371.
R. O. W. Fletcher and H. Steeple, Acta Crystallogr., 15 (1962) 960.
A. H. C. Ledsham and H. Steeple, Acta Crystallogr., Sect. B, 24 (1968) 320.
R. O. W. Fletcher and H. Steeple, Acta Crystallogr., 14 (1961) 891.
R. O. W. Fletcher and H. Steeple, Acta Crystallogr., 17 (1964) 290.
A. C. Larson and Don T. Cromer, Acta Crystallogr., 22 (1967) 793.
V. Jordanovska, Contrib. Mac. Acad. Sci. Arts, 12 (1991) 61.
V. Jordanovska, J. Thermal Anal., 35 (1989) 1331.
V. Jordanovska, Contrib. Mac. Acad. Sci. Arts, 13 (1992) 47.
V. Jordanovska and R. Trojko, Thermochim Acta, 228 (1993) 241.
V. Jordanovska, R. Trojko and B. Boyanov, Thermochim. Acta, 275 (1996) 301.
V. Jordanovska, B. Boyanov and P. Naumov, J. Therm. Anal. Cal., 66 (2001) 533.
N. Galesic and V. B. Jordanovska, Acta Cryst., C48 (1992) 256.
V. P. M. Pillai, V. U. Nayar and V. B. Jordanovska, Optics and Optoelectronic, 1 (1998) 193.
V. P. M. Pillai, V. U. Nayar and V. B. Jordanovska, Spectrochim. Acta, 56A (2000) 887.
V. Petrusevski and S. Aleksova, Croat. Chem. Acta, 64 (1991) 577.
C. Duval, Inorganic Thermogravimetric Analysis, Elsevier, Amsterdam 1963, p. 230.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jordanovska, V., Boyanov, B. & Pejov, L. Synthesis, characterisation and thermal decomposition of double sulfate. Journal of Thermal Analysis and Calorimetry 71, 629–634 (2003). https://doi.org/10.1023/A:1022876514932
Issue Date:
DOI: https://doi.org/10.1023/A:1022876514932