Abstract
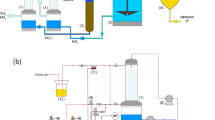
The wet scrubbing combined SOx/NO removal system is one ofthe advanced air pollution control devices. This study tries tounderstand the kinetics of the absorption in the system. The absorption of SO2 and simultaneous absorption of SO2 and NO, whose concentrations are typical for flue gases emittedfrom coal-fired power plants, in a stirred tank reactor with NaClO2/NaOH solutions were carried out at 50 °C.The liquid-side and gas-side mass transfer coefficients of the system were determined. The results indicate that the absorptionof SO2 is completely gas-film controlled if the NaOH concentration is greater than 0.1 M or the NaClO2 concentration is greater than 0.2 M. Adding SO2 would decrease the absorption rate of NO; however, the addition of NO has no effect on the absorption rate of SO2. The existence of O2 has no significant effect on the absorption rate of SO2 and NO in the combined SOx/NO removal tests.
Similar content being viewed by others
References
Bird, R. B., Stewart, W. E. and Lightfoot, E. N.: 1960, Transport phenomena, John Wiley and Sons, New York.
Chang, S. G. and Lee, G. C.: 1992, 'LBL PhoSNOX process for combined removal of SO2 and NOX from flue-gas', Environ. Prog. 11(1), 66–73.
Chien, T. W. and Chu, H.: 2000, 'Removal of SO2 and NO from flue gas by wet scrubbing using an aqueous NaClO2 solution', J. Hazard. Mater. 80(1- 3), 43–57.
Chien, T. W., Chu, H. and Hsueh, H. T.: 2001, 'Spray scrubbing of the nitrogen oxides into NaClO2 solution under acidic conditions', J. Environ. Sci. Health, Part A A36(4), 403–414.
Chu, H., Li, S. Y. and Chien, T. W.: 1998, 'The absorption kinetics of NO from flue gas in a stirred tank reactor with KMnO4/NaOH solutions', J. Environ. Sci. Health, Part A A33(5), 801–827.
Chu, H., Chien, T. W. and Li, S. Y.: 2001a, 'Simultaneous absorption of SO2 and NO from flue gas with KMnO4/NaOH solutions', Sci. Total Environ. 275(1- 3), 127–135.
Chu H., Chien T. W. and Twu, B. W.: 2001b, 'The absorption kinetics of NO in NaClO2/NaOH solutions', J. Hazard. Mater. 84(2- 3), 241–252.
Kobayashi, H., Takezawa, N. and Niki, T.: 1977, 'Removal of nitrogen oxides with aqueous solutions of inorganic and organic reagents', Environ. Sci. Technol. 11(2), 190–192.
Li, L.: 1993, 'Nitrogen Oxides Removal Using Chemical Oxidation Techniques', M. E. S., Lamar University - Beaumont.
Makansi, J.: 1990, 'Will combined SO2/NOx processes find a niche in the market?', Power 134(9), 26–28.
Onda, K., Sada, E., Kobayashi, T., Kito, S. and Ito, K.: 1970a, 'Salting-out parameters of gas solubility in aqueous solutions', J. Chem. Eng. Japan 3(1), 18–24.
Onda, K., Sada, E., Kobayashi, T., Kito, S. and Ito, K.: 1970b, 'Solubility of gases in aqueous solutions of mixed salts', J. Chem. Eng. Japan 3(2), 137–142.
Pham, E. K. and Chang, S. G.; 1994, 'Removal of NO from flue-gases by absorption to an iron(II) thiochelate complex and subsequent reduction to ammonia', Nature 369(6476), 139–141.
Sada, E., Kumazawa, H., Kudo, I. and Kondo, T.: 1978a, 'Absorption of NO in aqueous mixed solution of NaClO2 and NaOH absorption of NO in aqueous mixed xolution of NaClO2 and NaOH', Chem. Eng. Sci. 33, 315–318.
Sada, E., Kumazawa, H., Kudo, I. and Kondo, T.: 1978b, 'Kinetics of absorption of sulfur dioxide and nitric oxide in aqueous mixed solutions of sodium chlorite and sodium hydroxide', J. Chem. Eng. Japan 11(4), 276–282.
Sada, E., Kumazawa, H. and Hikosaka, H.: 1986, 'A kinetic study of absorption of NO into aqueous solutions of Na2SO3 with added FeII-EDTA chelate', Ind. Eng. Chem. Fundam 25(3), 386–390.
Shi, Y., Littlejohn, D., Kettler, P. B. and Chang, S. G.: 1996a, 'Removal of nitric oxide from flue gas with iron thiochelate aqueous solution in a turbulent contact absorber', Environ. Prog. 15(3), 153–158.
Shi, Y., Littlejohn, D. and Chang, S. G.: 1996b, 'Integrated tests for removal of nitric: oxide with iron thiochelate in wet flue gas desulfurization systems', Environ. Sci, Technol. 30(11), 3371–3376.
Shi, Y., Littlejohn, D. and Chang, S. G.: 1996c, 'Kinetics of NO absorption in aqueous iron(II) bis(2,3-dimercapto-1-propanesulfonate) solutions using a stirred reactor', Ind. Eng. Chem Res. 35(5), 1668–1672.
Shi, Y., Wang, H. and Chang, S. G.: 1997, 'Kinetics of NO absorption in aqueous iron (II)thiochelate solutions', Environ. Prog. 16(4), 301–306.
Teramoto, M., Ikeda, M. and Teranishi, H.: 1976, 'Absorption Rates of NO in Mixed Aqueous Solutions of NaClO2 and NaOH', Japanese Chem. Eng. 2(6), 637–640. (in Japanese).
Yang, C.-L.: 1994, 'Aqueous Absorption of Nitrogen Oxides Induced by Oxychlorine Compounds: A Process Development Study for Flue Gas Treatment', Ph.D. thesis, Dept. of Chemical Engineering, Chemistry and Environmental Science, New Jersey Institute of Technology.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chu, H., Chien, TW. & Twu, BW. Simultaneous Absorption of SO2 and NO in a Stirred Tank Reactor with NACLO2/NAOH Solutions. Water, Air, & Soil Pollution 143, 337–350 (2003). https://doi.org/10.1023/A:1022838623521
Issue Date:
DOI: https://doi.org/10.1023/A:1022838623521