Abstract
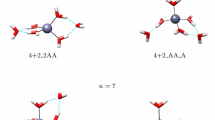
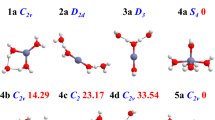
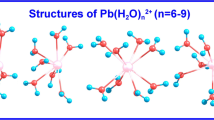
Raman spectra of aqueous Zn(II)–perchlorate solutions were measured over broad concentration (0.50–3.54 mol-L−1) and temperature (25–120°C) ranges. The weak polarized band at 390 cm−1 and two depolarized modes at 270 and 214 cm−1 have been assigned to ν1(a 1g), ν2(e g), and ν5(f 2g) of the zinc–hexaaqua ion. The infrared-active mode at 365 cm−1 has been assigned to ν3(f 1u). The vibrational analysis of the species [Zn(OH2) +2 ] was done on the basis of O h symmetry (OH2 as point mass). The polarized mode ν1(a 1g)-ZnO6 has been followed over the full temperature range and band parameters (band maximum, full width at half height, and intensity) have been examined. The position of the ν1(a 1g)-ZnO6 mode shifts only about 4 cm−1 to lower frequencies and broadens by about 32 cm−1 for a 95°C temperature increase. The Raman spectroscopic data suggest that the hexaaqua–Zn(II) ion is thermodynamically stable in perchlorate solution over the temperature and concentration range measured. These findings are in contrast to ZnSO4 solutions, recently measured by one of us, where sulfate replaces a water molecule of the first hydration sphere. Ab initio geometry optimizations and frequency calculations of [Zn(OH2) +2 ] were carried out at the Hartree–Fock and second-order Møller–Plesset levels of theory, using various basis sets up to 6-31 + G*. The global minimum structure of the hexaaqua–Zn(II) species corresponds with symmetry T h. The unscaled vibrational frequencies of the [Zn(OH2) +2 ] are reported. The unscaled vibrational frequencies of the ZnO6, unit are lower than the experimental frequencies (ca. 15%), but scaling the frequencies reproduces the measured frequencies. The theoretical binding enthalpy for [Zn(OH2) +2 ] was calculated and accounts for ca. 66% of the experimental single-ion hydration enthalpy for Zn(II).Ab initio geometry optimizations and frequency calculations are also reported for a [Zn(OH2) 182 ] (Zn[6 + 12]) cluster with 6 water molecules in the first sphere and 12 in the second sphere. The global minimum corresponds with T symmetry. Calculated frequencies of the zinc [6 + 12] cluster correspond well with the observed frequencies in solution. The ν1-ZnO6 (unscaled) mode occurs at 388 cm−1 almost in perfect correspondence to the experimental value. The theoretical binding enthalpy for [Zn(OH2) 182 ] was calculated and is very close to the experimental single ion-hydration enthalpy for Zn(II). The water molecules of the first sphere form strong hydrogen bonds with water molecules in the second hydration shell because of the strong polarizing effect of the Zn(II) ion. The importance of the second hydration sphere is discussed.
Similar content being viewed by others
REFERENCES
D. E. Irish and M. H. Brooker, Advances in Infrared and Raman Spectroscopy, Vol. 2 R. J. H. Clark and R. E. Hester, Eds., (Heyden, London, 1976), p. 212.
M. H. Brooker, in The Chemical Physics of Solvation, Part B. Spectroscopy of Solvation, R. R. Dogonadze, E. Kalman, A. A. Kornyshev, and J. Ulstrup, eds. (Elsevier, Amsterdam, 1986), p. 119.
M. Moskovits, Proc. 5th Int. Conf. Raman Spectrosc., Freiburg, Germany, 2–8 September, 1976, p. 768.
K. H. Michaellian, M. Moskovits, Nature 273, 135 (1978).
C. P. Nash, T. C. Donnelly, and P. A. Rock, J. Solution Chem. 6, 663 (1977).
J. T. Bulmer, D. E. Irish, and L. Oedberg, Can. J. Chem. 53, 3806 (1975).
D. E. Irish and T. Jarv, Chem. Soc. Faraday Discuss., pp. 64, 95, and 120 (1978).
F. Rull, Ch. Balarev, J. L. Alvarez, F. Sobron, and A. Rodriguez, J. Raman Spectrosc. 25, 933 (1994).
G. E. Walrafen, J. Chem. Phys. 36, 1035 (1962) J. Chem. Phys. 44, 1546 (1966).
W. Rudolph, M. H. Brooker, and C. C. Pye, J. Phys. Chem. 99, 3793 (1995).
C. C. Pye, W. Rudolph, and R. A. Poirier, J. Phys. Chem. 100, 601 (1996).
M. H. Brooker, O. Faurskov Nielsen, and E. Praestgaard, J. Raman Spectrosc. 19, 71 (1988).
A. I. Vogel, A Textbook of Quantitative Inorganic Analysis, 3rd edn (Longmans, London, 1961).
W. Rudolph and G. Irmer, J. Solution Chem. 23, 663 (1994).
W. W. Rudolph, Z. Phys. Chem. 194, 73 (1996).
W. W. Rudolph, M. H. Brooker, and P. R. Tremaine, Z. Phys. Chem., 209, 181 (1999).
W. W. Rudolph, M. H. Brooker, and P. R. Tremaine, J. Solution. Chem. 26, 757 (1997).
W. J. Hehre, R. F. Stewart, and J. A. Pople, J. Chem. Phys. 51, 2657 (1969); (b) W. J. Pietro and W. J. Hehre, J. Comput. Chem. 4, 241 (1983).
J. S. Binkley, J. A. Pople, and W. J. Hehre, J. Amer. Chem. Soc. 102, 939 (1980); (b) K. D. Dobbs and W. J. Hehre, J. Comp. Chem. 8, 880 (1987).
W. J. Hehre, R. Ditchfield, and J. A. Pople, J. Chem. Phys. 56, 2257 (1972).
S. Huzinaga, Gaussian Basis Sets for Molecular Calculations (Elsevier, Amsterdam, 1985).
R. A. Poirier, M. R. Peterson, and A. Yadav, Mungauss Chemistry Department, Memorial University of Newfoundland, St. John's, Newfoundland.
W. C. Davidon and L. Nazareth, Technical Memos 303 and 306, Applied Mathematics Division, Argonne National Laboratories, Argonne, IL, 1977. The algorithm is described in: W. C. Davidon, Math. Prog. 9, 1 (1975).
P. Cs'asz'ar and P. Pulay, J. Mol. Struct. 114, 31 (1975).
M. W. Schmidt, K. K. Baldridge, J. A. Boatz, S. T. Elbert, M. S. Gordon, J. H. Jensen, S. Koseki, N. Matsunaga, K. A. Nguyen, S. J. Su, T. L. Windus, M. Dupuis, and J. A. Montgomery, GAMES, Iowa State University, 17 July, 1993, Version, J. Comp. Chem. 14, 1347 (1993).
M. J. Frisch, G. W. Trucks, H. B. Schlegel, P. M. W. Gill, B. G. Johnson, M. W. Wong, J. B. Foresman, M. A. Robb, M. Head-Gordon, E. S. Replogle, R. Gomperts, J. L. Andres, K. Raghavachari, J. S. Binkley, C. Gonzalez, R. L. Martin, D. J. Fox, D. J. Defrees, J. Baker, J. J. P. Stewart, and J. A. Pople, Gaussian 92/DFT, Revision F.4, Gaussian, Inc., Pittsburgh, Pennsylvania, 1993.
C. I. Ratcliffe and D. E. Irish, Can. J. Chem. 62, 1134 (1984).
W. Rudolph and S. Schoenherr, Z. Phys. Chem. 270, 1121 (1989); (b) Z. Phys. Chem. 172, 31 (1991).
D. E. Irish and T. Jarv, J. Chem. Soc. Faraday Discuss., pp. 64, 95, and 120 (1978).
H. Kanno and J. Hiraishi, J. Raman Spectrosc. 9, 85 (1980); (b) H. Kanno, J. Phys. Chem. 92, 4232 (1988).
T. E. Jenkins and J. Lewis, Spectrochim. Acta 37, 47 (1981).
D. W. James and J. M. Whitnall, J. Raman Spectrosc., 7, 225 (1978).
A. Laubereau, G. Wochner, W. Kaiser, W., Chem Phys. 28, 363 (1978), and references therein.
K. S. Schweizer and D. Chandler, J. Chem. Phys. 76, 2296 (1982).
S. M. George, H. Auweter and C. B. Harris, J. Chem. Phys. 73, 5573 (1980), and references therein.
J. E. Griffiths, M. Clerc, and P. M. Rentzepis, J. Chem. Phys. 60, 3824 (1974).
S. Petrucci, in Ionic Interactions 8, Vol. II (Academic Press, New York, 1971), Chap. 7, p. 39ff.
W. W. Rudolph, Ber. Bunsenges. Phys. Chem. 102, 183 (1998); (b) W. W. Rudolph and C. C. Pye, J. Phys. Chem. B, 102, 3564 (1998).
S. Lee, J. K. Kim, J. K. Park, and K. S. Kim, J. Phys. Chem. 100, 14329 (1996).
C. C. Pye and W. W. Rudolph, J. Phys. Chem. A, 102, 9933 (1998).
M. Pavlov, P. E. M. Siegbahn, and M. Sandström, J. Phys. Chem. A, 102, 219 (1998).
N. Ohtomo, K. Arakawa, M. Takeuchi, T. Yamaguchi, and H. Ohtaki, Bull. Chem. Soc. Jpn. 54, 1314(1981); cf. also Y. Marcus, Chem. Rev. 88, 1475 (1988).
R. Åkesson, L. G. M. Petterson, M. Sandström, and U. Wahlgreen, J. Amer. Chem. Soc. 116, 8693 (1994) (cf. p. 8697, Table 4).
R. R. Pappalardo and E. S. Marcos, J. Phys. Chem. 97, 4500 (1993).
Muñoz-Paez, R. R. Pappalardo, and E. S. Marcos, J. Amer. Chem. Soc. 117, 11710 (1995).
G. Licheri, G. Paschina, G. Piccaluga, and G. Pinna, Z. Naturforsch. 37a, 1205 (1982).
C. C. Pye, unpublished results, 1998.
E. D. Glendening and D. Feller, J. Phys. Chem. 99, 3060 (1995); (b) D. Feller, E. D. Glendening, R. A. Kendall, and K. A. Peterson, J. Chem. Phys. 100, 4981 (1994).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Rudolph, W.W., Pye, C.C. Zinc(II) Hydration in Aqueous Solution: A Raman Spectroscopic Investigation and An ab initio Molecular Orbital Study of Zinc(II) Water Clusters. Journal of Solution Chemistry 28, 1045–1070 (1999). https://doi.org/10.1023/A:1022673226331
Issue Date:
DOI: https://doi.org/10.1023/A:1022673226331