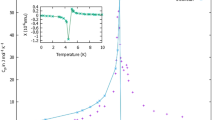
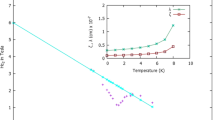
Abstract
Using only two principles: (i) high-temperature superconductivity requires hypercharged oxygen, and (ii) the superconducting condensates are located in those parts of the crystal structures where they are unaffected by magnetic pair breaking, we are able to explain why certain rare-earth ions R are compatible with superconductivity and others are not, in the compounds RBa2Cu3O7, RBa2Cu4O8, RBa2Cu2NbO8, R2 − z Ce z CuO4, and R2 − z Ce z Sr2Cu2NbO10. Various defects are proposed as having central roles in the superconductivity or the suppression of superconductivity in these compounds. Many experiments for testing this physical picture are suggested.
Similar content being viewed by others
REFERENCES
H. A. Blackstead and J. D. Dow, Phys. Rev. B 51, 11830 (1995).
H. A. Blackstead, J. D. Dow, A. K. Heilman, and D. B. Pulling, Solid State Commun. 103, 581 (1997).
H. A. Blackstead, J. D. Dow, I. Felner, H.-h. Luo, and W. B. Yelon, “Evidence of granular superconductivity in Pr2−z CezSr2Cu2NbO10,” Int. J. Mod. Phys. B, in press. In addition, H. Y. Hwang, S.-W. Cheong, A. S. Cooper, L. W. Rupp, Jr., B. Batlogg, G. H. Kwei, and Z. Tan, Physica 192, 362 (1992), have shown that Sr is easily soluble on Pr sites in the sister compound Pr2−z CezCuO4.
H. A. Blackstead, D. B. Chrisey, J. D. Dow, J. S. Horwitz, A. E. Klunzinger, and D. B. Pulling, Phys. Lett. A 207, 109 (1995); H. A. Blackstead, D. B. Chrisey, J. D. Dow, J. S. Horwitz, A. E. Klunzinger, and D. B. Pulling, Physica C 235–240, 1539 (1994).
H. A. Blackstead, J. D. Dow, D. B. Chrisey, J. S. Horwitz, P. J. McGinn, M. A. Black, A. E. Klunzinger, and D. B. Pulling, Phys. Rev. B 54, 6122 (1996).
Z. Zou, K. Oka, T. Ito, and Y. Nishihara, Jpn. J. Appl. Phys. Lett. 36, L18 (1997).
K. Oka, Z. Zou, and T. Ito, Physica C 282–287, 479 (1997).
M. Luszczek, W. Sadowski, and J. Olchowik, Abstract in 5th International Conference on Materials and Mechanisms of Superconductivity: High Temperature Superconductors, Abstract Book, p. 143 (1997); W. Sadowski, M. Luszczek, J. Olchowik, B. Susla, and R. Czajka, Molec. Phys. Rpts. 20, 213 (1997).
W. L. Hults, J. C. Cooley, E. J. Peterson, H. A. Blackstead, J. D. Dow, and J. L. Smith, “PrBa2Cu3O7 polycrystalline superconductor preparation,” Int. J. Mod. Phys. B, in press.
A. I. Romanenko and L. P. Kozeeva, Phys. Lett. A 223, 132 (1996).
H. A. Blackstead, J. D. Dow, D. Goldschmidt, and D. B. Pulling, “Observation of superconductivity in Gd2−z CezSr2Cu2TiOx,” to be published.
T. Usagawa, Y. Ishimaru, J. Wen, T. Utagawa, S. Koyama, and Y. Enomoto, Jpn. J. Appl. Phys. 36, L1583 (1997).
H. A. Blackstead, J. D. Dow, D. Goldschmidt, and D. B. Pulling, “Observation of predicted superconductivity in Gd2−z CezSr2Cu2TiOx with x≈10,” Phys. Lett. A (in press).
R2−z CezSr2Cu2TaO10 has virtually the same properties as R2−z CezSr2Cu2NbO10. Likewise, RBa2Cu2TaO8 and RBa2Cu2NbO8 are very similar. See [16]. R2−z CezSr2Cu2RuO10 has also been fabricated [17] but appears to be somewhat different from its Nb and Ta counterparts, and will be discussed in our subsequent work.
T. J. Goodwin, H. B. Radousky, and R. N. Shelton, Physica C 204, 212 (1992).
L. Bauerinfeld, W. Widder, and H. F. Braun, Physica C 253, 151 (1995).
S. K. Malik, H. Jhans, W. B. Yelon, and J. J. Rhyne, Solid State Commun. 85, 849 (1993).
H. Jhans, S. K. Malik, and R. Vijayaraghavan, Solid State Commun, 85, 105 (1993).
H. Jhans, S. K. Malik, and R. Vijayaraghavan, Physica C 215, 181 (1993).
M. Bennahmias, J. C. O'Brien, H. B. Radousky, T. J. Goodwin, P. Klavins, J. M. Link, C. A. Smith, and R. N. Shelton, Phys. Rev. B 46, 11986 (1992).
Z. Fisk, J. D. Thompson, E. Zirngiebl, J. L. Smith, and S. W. Cheong, Solid State Commun. 62, 743 (1987); P. Hor, R. L. Meng, Y.-Q. Wang, L. Gao, Z. J. Huang, J. Bechtold, K. Forster, and C. W. Chu, Phys. Rev. Lett. 58, 1891 (1987).
I. Felner and B. Brosh, Phys. Rev. B 43, 10364 (1991). T c0 = 82 K.
For a review, see H. B. Radousky, J. Mater. Res. 7, 1917 (1992).
H. A. Blackstead and J. D. Dow, Superlatt. Microstruct. 14, 231 (1993).
L. Soderholm, G. L. Goodman, U. Welp, C. W. Williams, and J. Bolender, Physica C 161, 252 (1989).
H. A. Blackstead and J. D. Dow, J. Supercond. 8, 613 (1995).
C. H. Pennington and C. P. Slichter, in Physical Properties of High-Temperature Superconductors, D. M. Ginsberg, ed. (World Scientific, Singapore), Vol. II, p. 269 et seq. (1990), especially p. 285, and references therein.
Ionization Potentials of Atoms and Atomic Ions, in Chemical Rubber Company Handbook, 74th edn., D. R. Lide, ed. (Chemical Rubber Publishing Company, Bombay, 1993–1994), pp. 10-205.
See, for example, H. Ledbetter and M. Lei, Physica C 166, 483 (1990).
We include the BaO layers as being in the “vicinity” of the CuO chains of the YBa2Cu3O7 and YBa2Cu4O8 homologues.
C. Q. Jin, Y. S. Yao, B. Q. Wu, Y. F. Xu, S. C. Liu, and W. K. Wang, Appl. Phys. Lett. 59, 3479 (1991).
Y. Tokura, H. Takagi, and S. Yoshida, Nature 337, 345 (1989).
A. Manthiram and Y. T. Zhu, Physica C 226, 165 (1994).
C. Lin, G. Lu, Z.-X. Liu, and Y. F. Zhang, Physica C 194, 66 (1992).
T. H. Meen, H. D. Yang, W. J. Huang, Y. C. Chen, W. H. Lee, J. H. Shieh, and H. C. Ku, Physica C 260, 117 (1996).
Y. K. Tao, M. Bonvalot, Y. Y. Sun, R. L. Meng, P. H. Hor, and C. W. Chu, Physica C 165, 13 (1990).
Y. K. Tao, Y. Y. Sun, J. Paredes, P. H. Hor, and C. W. Chu, J. Solid State Chem. 82, 176 (1989).
J. T. Markert, J. Beille, J. J. Neumeier, E. A. Early, C. L. Seaman, T. Moran, and M. B. Maple, Phys. Rev. Lett. 64, 80 (1990).
J. T. Markert, E. A. Early, T. Bjørnholm, S. Ghamaty, B. W. Lee, J. J. Neumeier, R. D. Price, C. L. Seaman, and M. B. Maple, Physica C 158, 178 (1989).
H. Ishii, T. Koshizawa, T. Hanyu, and S. Yamaguchi, Jpn. J. Appl. Phys. 32, 1070 (1993).
W. J. Zhu, Y. S. Yao, X. J. Zhou, B. Yen, C. Dong, Y. Z. Huang, and Z. X. Zhao, Physica C 230, 385 (1994).
C. L. Seaman, N. Y. Ayoub, T. Bjørnholm, E. A. Early, S. Ghamaty, B. W. Lee, J. T. Markert, J. J. Neumeier, P. K. Tsai, and M. B. Maple, Physica C 159, 391 (1989).
Pr2−z ThzCuO4, Nd2−z ThzCuO4 [43], and Tm2−z CazCuO4 [42] also assume the T′ crystal structure of Nd2−z CezCuO4, and superconduct.
To our knowledge, the highest reported values of Tc for the T′ structure R2−z CezCuO4 superconductors are: 24.3 K for R = Pr, z = 0.10 [47], 21.2 K for R = Nd, z = 0.15 [39] or 32 K for pressure-fabricated material [32], 19.7 K for R = Sm, z = 0.15 [39], 7.5 K for R = Eu, z = 0.15 [36], 21 K for La0.28Nd1.57Ce0.15CuO4 [35], 47 K and 18.5 K for La0.95Eu0.95Ce0.10CuO4 [48], and La01.22Eu0.63Ce0.15CuO4 [46], respectively. In contrast, LaGd0.8Ce0.2CuO4 does not superconduct [37], nor does Nd1.85−x GdxCe0.15CuO4 for 1.1 ≤ x ≤ 1.85 [35,49], although Gd is soluble over the entire range 0 < x < 1.85.
M. Yoshimoto, H. Koinuma, T. Hashimoto, J. Tanaka, S. Tanabe, and N. Soga, Physica C 181, 284 (1991).
M. Brinkmann, T. Rex, M. Stief, H. Bach, and K. Westerhalt, Physca C 269, 76 (1996).
H. Itoh, M. Kusuhashi, and M. Taniwaki, Jpn. J. Appl. Phys. 29, L1604 (1990).
G. H. Hwang, J. H. Shieh, and H. C. Ku, Physica C 185–189, 1163 (1991).
Rukang Li, Yingjie Zhu, Yitai Qian, and Zuyao Chen, Physica C 176, 19 (1991); Rukang Li, Yingjie Zhu, Cheng Xu, Zuyao Chen, Yitai Qian, and Chengao Fan, J. Solid State Chem. 94, 206 (1991).
A. J. Wright, R. A. Jennings, and C. Greaves, Supercond. Sci. Technol. 6, 514 (1993).
H. W. Zandbergen, R. J. Cava, J. J. Krajewski, and W. F. Peck, Jr., Physica C 192, 223 (1992); H. W. Zandbergen, R. J. Cava, J. J. Krajewski, and W. F. Peck, Jr., Physica C 196, 252 (1992).
R. J. Cava, J. J. Krajewski, H. Takagi, H. W. Zandbergen, R. B. Van Dover, W. F. Peck, Jr., and V. Hessen, Physica C 191, 237 (1992).
Shiwei Wang, Yitai Qian, Rukang Li, Zuyao Chen, Nanlin Wang, Liezhao Cao, Guien Zhou, and Yuheng Zhang, Physica C 210, 463 (1993).
H. A. Blackstead and J. D. Dow, J. Appl. Phys. 83, 1536 (1998).
H. A. Blackstead and J. D. Dow, Phys. Rev. B 57, 10798 (1998).
H. A. Blackstead and J. D. Dow, J. Appl. Phys. 83, 1540 (1998).
H. A. Blackstead and J. D. Dow, “Comparison of bulk R2−z CezCuO4 with superlattice R2−z CezCuO4/SrO/NbO2/SrO/CuO2/,” unpublished.
An alternative explanation of the different nature of Pr in P2−z CezCuO4 only [60,61] is based on its nonmagnetic singlet ground state. See [62–64]. This issue deserves more study.
G. Cao, J. Bolivar, J. W. O'Reilly, J. E. Crow, R. J. Kennedy, and P. Pernambuco-Wise, Physica B 186–188, 1004 (1993).
H. B. Radousky, T. J. Goodwin, and R. N. Shelton, Physica C 209, 155 (1993).
P. Allenspach, S.-W. Cheong, A. Dommann, P. Fischer, Z. Fisk, A. Furrer, H. R. Ott, and B. Rupp, Z. Phys. B 77, 185 (1989).
M. Matsuda, K. Yamada, K. Kakirai, H. Kadowaki, T. R. Thurston, Y. Endoh, Y. Hidaki, R. J. Birgeneau, M. A. Kastner, P. M. Gehring, A. H. Moudden, and G. Shirane, Phys. Rev. B 42, 10098 (1990).
G. L. Goodman, C. K. Loong, and L. Soderholm, J. Phys. Cond. Matter 3, 49 (1991).
H. A. Blackstead and J. D. Dow, J. Appl. Phys. 81, 6285 (1997).
H. A. Blackstead and J. D. Dow, Philos. Mag. B 72, 529 (1995).
T. H. Meen, H. D. Yang, W. J. Huang, Y. C. Chen, W. H. Lee, J. H. Hsieh, and H. C. Ku, Physica C 260, 117 (1996).
The critical concentrations of Gd required to drive Tc to zero in La2−βSrβCuO4 and Nd2−zCezCuO4 are uc≈0.38 and u′c≈1.13, respectively. The corresponding concentration for Ni-doped Nd2−z CezCuO4 is u″c = 0.0067. The condition for driving Tc to zero is roughly proportional to [69] the rates ФΞuc exp(−r/R Gd), Ф′Ξu′c exp(−r′/R Gd), and Ф″Ξu″c exp(−r″/R Ni) (using r≈2.69 Å and r′≈2.04 Å for the La-OI and Nd-OI distances, where OI is interstitial oxygen, and r″≈1.48 Å for the Cu-OI distance, and taking R Gd = 0.4 Å and R Ni = 0.7 Å). We find ratios of these rates (Ф/Ф′ and Ф″/Ф′) of the same order of magnitude for Gd and Ni doping: 0.5–8. This is a crude indication that the degradation of Tc in Gd-doped T and T′ structures and in Ni-doped Nd2−z CezCuO4 are all due to the breaking of pairs in the charge reservoirs: the range of values of uc exp(−r/R) is reduced by more than an order of magnitude from the range of critical compositions uc.
A. A. Abrikosov and L. P. Gor'kov, Zh. Eksp. Teor. Fiz. 39, 1781 (1960); [English transl.: Soviet Phys. JETP 12, 1243–1254 (1961)]. See also [71].
O. Peña and M. Sargent, Prog. Solid State Chem. 19, 165 (1989).
Y.-J. Kim and A. W. Overhauser, Phys. Rev. B 49, 15799 (1994).
I. D. Brown and D. Altermatt, Acta Cryst. B 41, 244 (1985); D. Altermatt and I. D. Brown, Acta Cryst. B 41, 241 (1985); I. D. Brown, J. Solid State Chem. 82, 122 (1989). I. D. Brown and K. K. Wu, Acta Cryst. B 32, 1957 (1976); I. D. Brown, Structure and Bonding in Crystals, Vol. II, M. O'Keefe and A. Navrotsky, eds. (Academic Press, New York, 1980), pp. 1–20.
H. A. Blackstead and J. D. Dow, J. Superconduct. 9, 563 (1996).
H. A. Blackstead and J. D. Dow, J. Appl. Phys. 78, 7175 (1995).
J. C. Phillips, Phys. Rev. B 41, 8968 (1990) has advanced the viewpoint that the superconductivity originates in percolative paths between the CuO chains and the cuprate planes.
I. Bozovic and J. N. Eckstein, “Superconductivity in cuprate superlattices,” in Physical Properties of High-Temperature Superconductors V, D. M. Ginsberg, ed. (World Scientific, Singapore, 1996).
J. M. Triscone, O. Fischer, L. Antognazza, A. D. Kent, and M. J. Karkut, Phys. Rev. Lett. 64, 804 (1990).
L. Soderholm, C. W. Williams, and U. Welp, Physica C 179, 440 (1991).
Ca doping of RBa2Cu2NbO8 should be feasible, but could produce hypocharged oxygen in the NbO2 layers. Therefore the only tests capable of distinguishing between hypocharged oxygen and carriers (e.g., holes) as the generator of superconductivity are the ones that dope the material n-type.
The bond-charge method [81] does not achieve self-consistency for any reasonable combination of Madelung potentials or ion charges in the cases of NdBa2Cu2NbO8 and NdBa2Cu2TaO8, although the Ta-based compound comes closer than the Nd-based compound. The most reasonable solutions of the model have Nb and Ta in the +5 charge states, but with the strength of the ionization potentials about 5 and 2 V too weak in magnitude for Nb and Ta, respectively. In both cases, the bond-valence-sum charge for Nd or Ta is essentially equal to +5|e|. The possibility of repairing this problem by considering Nb to be in the + 3 valence state (with seven oxygen ions instead of 8) appears remote: the magnitude of the ionization potential is too weak by ~2 V to form a Nb+3 state, and the stability of Ta in any charge-state except Ta+5 is questionable. This problem could be fixed by having the material adopt an off-stoichiometry configuration: increasing the number or charge of oxygen ions, or by increasing the number of cation vacancies. While there is evidence that these compounds prefer to form off-stoichiometry, especially the Nb variant, only these two insulators of the many materials we have studied have defied conforming to the self-consistent bond/charge model. One can speculate that some Schottky barrier or work function is responsible for the problem, and that screening would reduce it if the materials were metallic, but it is equally likely that the bond-valence-sum parameters for Nb and Ta need some revision.
H. A. Blackstead and J. D. Dow, Phys. Rev. B 55, 6605 (1997).
H. A. Blackstead and J. D. Dow, “Constraints imposed by the data on a successful theory of high-temperature superconductivity,” Proc. SPIE 2397, Optoelectronic Integrated Circuit Materials—Physics and Devices, M. Razeghi, Y.-S. Park, and G. L. Witt, eds. (1995), pp. 617-632; “High-temperature superconductivity,” in Proceedings of the Second International Symposium on Quantum Confinement Physics and Applications, M. Cahay, S. Bandyopadhyay, J. P. Leburton, A. W. Kleinsasser, and M. A. Osman, eds. (Electrochemical Society, Inc., Pennington, New Jersey, 1994), Vol. 94–17, pp. 408–418.
H. A. Blackstead and J. D. Dow, J. Phys. Chem. Solids 56, 1697 (1995); H. A. Blackstead, J. D. Dow, J. F. Federici, W. E. Packard, and D. B. Pulling, Physica C 235–240, 2161 (1994).
H. A. Blackstead and J. D. Dow, Physica C 265, 143 (1996).
R. J. Cava, A. W. Hewat, E. A. Hewat, B. Batlogg, M. Marezio, K. M. Rabe, J. J. Krajewski, W. F. Peck, Jr., and L. W. Rupp, Jr., Physica C 165, 419 (1990).
Y. Tokura, J. B. Torrance, T. C. Huang, and A. I. Nazzal, Phys. Rev. B 38, 7156 (1988).
M. J. Kramer, S. I. Yoo, R. W. McCallum, W. B. Yelon, H. Xie, and P. Allenspach, Physica C 219, 145 (1994).
H. A. Blackstead and J. D. Dow, Solid State Commun. 95, 613 (1995); “Implications of magnetic pair breaking in YBa2Cu3O7 homologues,” in Quantum Confinement III: Quantum Wires and Dots, M. Cahay, S. Bandyopadhyay, J.-P. Leburton, and M. Razeghi, eds. (Electrochemical Society, Inc., Pennington, New Jersey, 1990), pp. 339–354.
See, for example, M. Tinkham, Introduction to Superconductivity, 2nd edn. (McGraw-Hill, New York, 1996), p. 197.
B. V. Kresin, H. Morawitz, and S. A. Wolf, Mechanisms of Conventional and High-T c Superconductivity (Oxford, New York, 1993), pp. 103-127.
D. N. Basov, R. Liang, D. A. Bonn, W. N. Hardy, B. Dabrowski, M. Quijada, D. B. Tanner, J. P. Rice, D. M. Ginsberg, and T. Timusk, Phys. Rev. Lett. 74, 598 (1995).
L. Hoffmann, A. A. Manuel, M. Peter, E. Walker, M. Gauthier, A. Shukla, B. Barbiellini, S. Massidda, G. Adam, S. Adam, W. N. Hardy, and R. X. Liang, Phys. Rev. Lett. 71, 4047 (1993).
A. Shukla, L. Hoffmann, A. A. Manuel, E. Walker, B. Barbiellini, and M. Peter, Phys. Rev. B 51, 6028 (1995).
S. Sridhar, Bull. Am. Phys. Soc. 42, 698 (1997).
H. A. Blackstead and J. D. Dow, Pis'ma Zh. Eksp. Teor. Fiz. 59, 262 (1994) English transl.: [JETP Lett 59, 283 (1994)] and references therein.
A. Matushita, Y. Yamada, N. Yamada, S. Horii, and T. Matsumoto, Physica C 242, 381 (1995).
N. Seiji, S. Adachi, and H. Yamauchi, Physica C 227, 377 (1994).
G. Cao, R. J. Kennedy, J. W. O'Reilly, J. E. Crow, P. Pernambuco-Wise, and S. T. Ting, Physica B 186–188, 1022 (1993).
E. E. Alp, S. M. Mini, M. Ramanathan, B. Dabrowski, D. R. Richards, and D. G. Hinks, Phys. Rev. B 40, 2617 (1989).
R. G. Goodrich, C. Grienier, D. Hall, A. Lacerda, E. G. Haanappel, D. Rickel, T. Northington, R. Schwarz, F. M. Mueller, D. D. Koelling, J. Vuillemin, L. Van Bockstal, M. L. Norton, and D. H. Lowndes, J. Phys. Chem. Solids 54, 1251 (1993).
M. K. Wu, D. Y. Chen, F. Z. Chien, S. R. Sheen, D. C. Ling, C. Y. Tai, G. Y. Tseng, D. H. Chen, and F. C. Zhang, Z. Phys. B 102, 37 (1997).
J. Sichelschmidt, B. Elaschner, A. Loidl, and B. I. Kochelaev, Phys. Rev. B 51, 9199 (1995).
In low-defect YBa2Cu3Ox crystals that are incompletely oxygenated, such as for x≈0.8, electron-spin resonance has been observed over a limited temperature range (T > 80 K), which is thought to be associated with isolated Cu+2 ions at the ends of chain fragments.
See, for example, the discussion of Zhang-Rice singlets in F. C. Zhang and T. M. Rice, Phys. Rev. B 37, 3759 (1988).
See [106] for a discussion of the experimental inadequacy of Zhang-Rice singlets.
J. L. Sarrao, D. P. Young, Z. Fisk, E. G. Moshopoulou, J. D. Thompson, B. C. Chakoumakos, and S. E. Nagler, Phys. Rev. B 54, 12014 (1996), and to be published.
For example, molecular orbitals associated with Cu in CuO chains, but directed toward the BaO layers, or combinations of orbitals similar to those proposed for cuprate-plane superconductivity [59,61–64,104] might prove to be appropriate.
R. S. Liu, J. R. Cooper, J. W. Loram, W. Zhou, W. Lo, P. P. Edwards, W. Y. Liang, and L. S. Chen [Solid State Commun. 76, 679 (1990)] have reported 20 K superconductivity in p-type Ca-doped, severely deoxygenated YBa2 Cu3O6.1 with almost no CuO chain oxygen. This low-temperature superconductivity might be associated with BaO or even with cuprate planes, but appears to have a different origin from the superconductivity of YBa2Cu3Ox with x ≥ 6.5, especially when the deoxygenated material clearly has more holes than the higher-Tc superconductor, such as for x = 6.5. See also G. Böttger, I. Mangelschots, E. Kaldis, P. Fischer, Ch. Krüger, and F. Fauth, J. Phys. Cond. Matter 8, 8889 (1996).
If the rare-earth ion is crystal-field split [70], then it can be adjacent to the superconducting condensate and not break Cooper pairs. See [59,61,63,64,70].
Computed for the data of [111,112].
R. J. Cava, B. Batlogg, K. M. Rabe, E. A. Rietman, P. K. Gallagher, and L. W. Rupp, Jr., Physica C 156, 523 (1988).
J. D. Jorgensen, B. W. Veal, A. P. Paulikas, L. J. Nowicki, G. W. Crabtree, H. Claus, and W. K. Kwok, Phys. Rev. B 41, 1863 (1990).
In the major high-temperature superconductors, the charge reservoirs contain no Cu, except for nonmagnetic CuO chains (which have adjacent BaO or SrO layers) in the 123-type compounds and in R2−z CezSr2Cu2NbO10 homologues.
M. Park, M. J. Kramer, K. W. Dennis, and R. W. McCallum, Physica C 259, 43 (1996).
J. B. Barner, C. T. Rogers, A. Inam, R. Ramesh, and S. Bersey, Appl. Phys. Lett. 59, 742 (1991).
Y. Suzuki, J.-M. Triscone, C. B. Eom, M. R. Beasley, and T. H. Geballe, Phys. Rev. Lett. 73, 328 (1994); Y. Suzuki, J.-M. Triscone, M. R. Beasley, and T. H. Geballe, Phys. Rev. B 52, 6858 (1995).
P. R. Slater and C. Greaves, Supercond. Sci. Technol. 5, 205 (1992); A. Tokiwa, Y. Syono, M. Kikuchi, R. Suzuki, T. Kajitani, N. Kobayashi, P. Sasaki, O. Nakatso, and Y. Moto, Jpn. J. Appl. Phys. 27, L1009 (1988).
H. A. Blackstead, J. D. Dow, J. F. Federici, W. E. Packard, and D. B. Pulling, Physica C 235–240, 2161 (1994).
H. A. Blackstead and J. D. Dow, unpublished analysis of the data of [87].
H. A. Blackstead and J. D. Dow, J. Phys. Chem. Solids 56, 1697 (1995).
J. D. Jorgensen, B. W. Veal, A. P. Paulikas, L. J. Nowicki, G. W. Crabtree, H. Claus, and W. K. Kwok, Phys. Rev. B 41, 1863 (1990).
P. G. Radaelli, D. G. Hinks, A. W. Mitchell, B. A. Hunter, J. L. Wagner, B. Dabrowski, K. G. Vandervoort, H. K. Viswanathan, and J. D. Jorgensen, Phys. Rev. B 49, 4163 (1994).
A. J. Freeman (private communication) and co-workers have argued that the local state density at the Fermi energy is so low near the rare-earth site that the site is effectively magnetically isolated from the Fermi sea. However, the spin relaxation on that site is Korringa-like (indicating efficient coupling to the Fermi liquid), and the state-density at the Ba site is comparably low—and Nd on a Ba site certainly breaks pairs in NdBa2Cu3Ox. See A. J. Freeman, J. Yu, S. Massidda, and D. D. Koelling, Phys. Lett. A 122, 198 (1987); J. T. Markert, T. W. Noh, S. E. Russek, and R. M. Cotts, Solid State Commun. 63, 847 (1987).
H. A. Blackstead, J. D. Dow, W. E. Packard, and D. B. Pulling, Physica C 235–240, 1363 (1994).
P. Yossefov, G. E. Shter, G. M. Reisner, A. Friedman, Y. Yeshurun, and G. S. Grader [Physica C 275, 299 (1997)] have reported Tc≈98 K for NdBa2Cu3O7.
Our data [5] on granular materials suggest that the onset critical temperature of perfect PrBa2Cu3O7 might well be ≈98 K.
Of course, the superconducting PrBa2Cu3O7 of [4–11] can easily be explained as having their critical temperatures degraded by PrBa defects, but this requires that the condensate be in the vicinity of the CuO chains, not in the cuprate planes.
The ionic radii of Ca+2, Sr+2, Ba+2; Hf+4, Ce+4, Th+4; La+3, Ce+3, Pr+3, Nd+3, Sm+3, Eu+3, Gd+3, Y+3, and Tm+3 are (in Å): 0.99, 1.12, 1.34, 0.78, 0.94, 1.02, 1.14, 1.07, 1.06, 1.04, 1.00, 0.98, 0.97, 0.92, and 0.87, respectively. See F. S. Galasso, Structure, Properties, and Preparation of Perovskite-Type Compounds (Pergamon Press, Oxford, 1969), p. 52.
2.48 Å for PrBa2Cu3O7 [132]; 2.37 Å for PrBa2Cu4O8 [130]; and 2.69 Å for Pr2−z CezCuO4 with z = 0.15 [131].
Yuh Yamada, S. Horii, N. Yamada, Z. Guo, Y. Kodama, K. Kawamoto, U. Mizutani, and I. Hirabayashi, Physica C 231, 131 (1994).
J. Y. Markert, E. A. Early, T. Bjørnholm, S. Ghamaty, B. W. Lee, J. J. Neumeier, R. D. Price, C. L. Seaman, and M. B. Maple, Physica C 158, 178 (1989).
S. R. Malik, W. B. Yelon, J. J. Rhyne, W. R. James, R. Prasada, K. Adhikary, and N. C. Soni, Solid State Commun. 89, 383 (1994).
J. Ye, Z. Zou, A. Matsushita, K. Oka, Y. Nishihara, and T. Matsumoto, Phys. Rev. Lett. 80, 1074 (1998).
Y. Xu and W. Guan, Solid State Commun. 80, 105 (1991); Appl. Phys. Lett. 59, 2183 (1991); Physica C 183, 105 (1991); Appl. Phys. Lett. 59, 2183 (1991).
H. A. Blackstead and J. D. Dow, Phys. Lett. A 26, 97 (1997).
D. J. Scalapino, Phys. Rep. 250, 1 (1995).
J. Kondo, Y. Asai, and S. Nagai, J. Phys. Soc. Jpn. 57, 4434 (1988).
H. A. Blackstead and J. D. Dow, Superlatt. Microstruct. 17, 473 (1995).
L. Soderholm, C.-K. Loong, G. L. Goodman, and B. D. Dabrowski, Phys. Rev. B 43, 7923 (1991).
J. Bardeen, L. N. Cooper, and J. R. Schrieffer, Phys. Rev. 106, 162 (1957); 108, 1175 (1957).
The ionization potentials to the +2 charge state of Ni, Cu, and Zn are 18.2 V, 20.3 V, and 18.0 V [29].
The ionic radii of Ni+2, Cu+2, and Zn+2 are 0.69, 0.72, and 0.7 Å, respectively [128].
T. Yamashita, A. Kawakami, T. Nishihara, Y. Hirotsu, and M. Takata, Jpn. J. Appl. Phys. 26, L635 (1987); T. Yamashita, A. Kawakami, T. Nishihara, M. Takata, and K. Kishio, Jpn. J. Appl. Phys. 26, L671 (1987); T. J. Witt, Phys. Rev. Lett. 61, 1423 (1988); D. Esteve, J. M. Martinis, C. Urbina, M. H. Devoret, G. Collin, P. Monod, M. Ribault, and A. Revcolevschi, Europhys. Lett. 3, 1237 (1987); R. H. Koch, C. P. Umbach, G. J. Clark, P. Chaudhari, and R. B. Laibowitz, Appl. Phys. Lett. 51, 200 (1987); C. E. Gough, M. S. Colclough, E. M. Forgan, R. G. Jordan, M. Keene, C. M. Muirhead, A. I. M. Rae, N. Thomas, J. S. Asbell, and S. Sutton, Nature 326, 855 (1987); P. Gammel, D. J. Bishop, G. J. Dolan, J. R. Kwo, C. A. Murray, L. F. Schneemeyer, and J. V. Waszczak, Phys. Rev. Lett. 59, 2592 (1987).
We have recently become aware of a dissenting view [145,147] concerning these facts, especially for YBa2Cu4O8. Compare the results reported by these authors with those of [146], while paying particular attention to the evidence that the impurities occupy the same Cu sites.
S. K. Agarwal and A. V. Narlikar, Prog. Cryst. Growth Charact. 28, 219 (1994).
H. A. Blackstead and J. D. Dow, Philos. Mag. B 74, 681 (1996).
G.-q. Zheng, T. Odaguchi, Y. Kitaoka, K. Asayama, Y. Kodama, K. Mizuhashi, and S. Uchida, Physica C 263, 367 (1996).
D. Pines, in Strongly Correlated Electronic Materials: Los Alamos Symposium 1993, K. Bedell, Z. Wang, D. E. Meltzer, A. Valatsky, and E. Abrahams, eds. (Addison-Wesley, Reading, Massachusetts, 1994).
H. A. Blackstead and J. D. Dow, “Self-test of spin-fluctuation pairing,” Europhys. Lett., in press.
F. Bridges, J. B. Boyce, and R. I. Johnson, Mater. Res. Soc. Symp. 275, 143 (1992); A. M. Balagurov, J. Piechota, and A. Pajaczkowska, Solid State Commun. 78, 407 (1991).
F. Bridges, J. B. Boyce, T. Claeson, T. H. Geballe, and J. M. Tarascon, Phys. Rev. B 42, 2137 (1990); F. Bridges, G. G. Li, J. B. Boyce, and T. Claeson, Phys. Rev. B 48, 1266 (1993).
D. Goldschmidt, Y. Direktovitch, A. Knizhnik, and Y. Eckstein, Phys. Rev. B 54, 13348 (1996).
J. E. Hirsch and F. Marsiglio, Physica C 162–164, 591 (1989).
D. I. Khomskii and A. Z. Zvezdin, Solid State Commun. 66, 651 (1988).
The Madelung potential at a Ce site in Nd2−z CezCuO4 is ≈ − 29.85 V.
The corresponding inscribed cage radii for R2−z CezSr2Cu2NbO10 are 1.036, 1.046, 1.034, and 1.043 Å, respectively, for R = Pr, Nd, Sm, and Eu, and are all smaller than the critical radius for Z slightly larger than unity. (Recall that some nonzero p-type doping is required, and that we have A(O−1) = 1.025 Å.)
In the R2−z CezSr2Cu2NbO10 compounds, the volumes of the interstitial cages are smaller than for Gd2−z CezCuO4's cage, and so we expect the interstitial not to form easily. See [57] H. A. Blackstead and J. D. Dow, Phys. Rev. B 57, 10798 (1998).
A. Nath, N. K. Kopelev, V. Chechersky, J.-L. Peng, R. L. Greene, B.-h. O, M. I. Larkin, and J. T. Markert, Science 265, 73 (1994).
P. G. Radaelli, J. D. Jorgensen, A. J. Schultz, J. L. Peng, and R. L. Greene, Phys. Rev. B 49, 15322 (1994).
M. Lehmann, private communication.
C. E. Kuklewicz and J. Markert, Physica C 253, 308 (1995).
The effective Eu moment decreases with temperature.
High-pressure fabrication can facilitate formation of Nd2−z CezCuO4 homologues by permitting rare-earth ions to form bonds that would not form without pressure. High-pressure oxygenation can insert more interstitial oxygen into the sample.
We are unable to find evidence in the literature that Tb2CuO4 has been fabricated or doped.
For R = Pr, Nd, Sm, and Eu, we find Z to be 1.68, 1.72, 1.78, and 1.76, respectively.
H. A. Blackstead and J. D. Dow, J. Supercond. 8, 613 (1995).
S. C. Cheng, V. P. Dravid, T. J. Goodwin, R. N. Shelton, and H. B. Radousky, Phys. Rev. B 53, 11779 (1996).
H. A. Blackstead and J. D. Dow, Philos. Mag. B 72, 529 (1995).
J. Arai, H. Shimizu, D. Yamaguchi, Physica C 185–189, 1205 (1991).
I. Mangelschots, N. H. Anderson, B. Lebech, A. Wisniewski, and C. S. Jacobsen, Physica C 203, 369 (1992).
S. N. Mao, X. X. Xi, Q. Li, L. Takeuchi, S. Bhattacharya, C. Kwon, C. Doughty, A. Walkenhorst, T. Venkatesan, C. B. Whan, J. L. Peng, and R. L. Greene, Appl. Phys. Lett. 62, 2425 (1993).
I. D. Brown and D. Altermatt, Acta Cryst. B 41, 244 (1985); D. Altermatt and I. D. Brown, Acta Cryst. B 41, 241 (1985); I. D. Brown, J. Solid State Chem. 82, 122 (1989). I. D. Brown and K. K. Wu, Acta Cryst. B 32, 1957 (1976); I. D. Brown, Structure and Bonding in Crystals, Vol. II, M. O'Keefe and A. Navrotsky, eds. (Academic Press, New York, 1980), pp. 1–20.
A. J. Schultz, J. D. Jorgensen, J. L. Peng, and R. L. Greene, Phys. Rev. B 53, 5157 (1996). See footnote 45 of [81] and P. Ghigna, G. Spinolo, M. Scavini, U. Anselmi Tamburini, and A. V Chadwick, Physica C 253, 147(1995).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Blackstead, H.A., Dow, J.D. Rare-Earths as Probes of High-Temperature Superconductivity. Journal of Superconductivity 11, 615–639 (1998). https://doi.org/10.1023/A:1022643531034
Issue Date:
DOI: https://doi.org/10.1023/A:1022643531034