Abstract
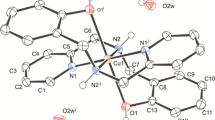
In the neutral title complex [Cu(C4N2H3)2(H2O)3] or [Cu(BBR)2(H2O)3] (BBR− = Barbiturate), the CuII ion, in the slightly distorted square-pyramidal geometry, is coordinated by two O atoms of the two monodentate barbiturates and three O atoms of three water ligands. The average bond length of Cu—O (BBR−) is 1.981(5) Å and the average bond length of Cu—O (H2O) at the basal sites is 1.94(5) Å, i.e. much shorter than that of Cu—O (H2O) [2.175(11) Å]. The crystal structure is characterized by an extensive network of hydrogen bonds in which each [Cu(BBR)2(H2O)3] entity links to six adjacent [Cu(BBR)2(H2O)3] by O(C=O) ··· H—O(H2O) bonds. Tautomerism in the coordination process for BBR− was found from the crystal structure and i.r. spectral analysis. The interaction of CuII and BBR− in aqueous solution was also investigated by electronic spectra and electrochemical method. It was observed that the copper ion could not only form the [Cu(BBR)2(H2O)3] complex in aqueous but also catalyze the decomposition of BBR− at pH 1.1.
Similar content being viewed by others
References
J.N. Delgado and W.A. Remers, J.B. Lippincott (ed) in Wilson and Gisvold's Textbook of Organic Medicinal and Pharmaceutical Chemistry, 9th Edit., Philadephia, 1991, pp. 39, 341, 376.
A. Wang and B.M. Craven, J. Pharm. Sci., 68, 361 (1979).
G.P. Jones and P.R. Andrews, J. Chem. Soc., Perkin Trans., 2, 415 (1987).
O. Simonsen, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., C41, 1258 (1985).
G.L. Gartland and B.M. Craven, Acta Crystallogr., Sect. B, 30, 980 (1974).
H.A.A. Medien and A.A. Zahran, Spectrochimica Acta Part A, 57, 2505 (2001).
J.A. Zerkowski, J.P. Mathias and G.M. Whitesides, J. Am. Chem. Soc., 116, 4305 (1994).
P.V. Bernhardt, Inorg. Chem., 38, 3481 (1999).
K.C. Russell, J.M. Lehn, N. Kyritsakas, A. DeCian and J. Fischer, New J. Chem., 22, 123 (1998).
J.A. Zerkowski, C.T. Seto, D.A. Wierda and GM. Whitesides, J. Am. Chem. Soc., 112, 9025 (1990).
J.M. Lehn, M. Mascal, A. DeCian and J. Fischer, J. Chem. Soc., Chem. Commun., 479 (1990).
B. Berking, Acta Crystallogr. Sect. B, 28, 98 (1972).
G.M. Shelddrick, SHELXTL PC, Program Package for X-ray Crystal Structure Determination. Siemens Analytical X-ray instruments Inc., Karlsruhe, Germany, 1990.
W. Bolton, Acta Cryst., 16, 950 (1963).
A.R. Al-Karaghouli, B. Abdul-Wahab E. Ajaj and S. Al-Asaff, Acta Cryst., B33, 1655 (1977).
B.M. Craven, Acta Cryst., 16, 282 (1964).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Xiong, Y., He, C., An, TC. et al. An approach to the structure and spectra of copper barbiturate trihydrate. Transition Metal Chemistry 28, 69–73 (2003). https://doi.org/10.1023/A:1022592701631
Issue Date:
DOI: https://doi.org/10.1023/A:1022592701631