Abstract
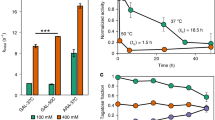
L-arabinose isomerase (EC 5.3.1.4) mediates the isomerization of D-galactose into D-tagatose as well as the conversion of L-arabinose into L-ribulose. To investigate the properties of L-arabinose isomerase as a biocatalyst for the conversion of galactose to tagatose, the L-arabinose isomerase of Escherichia coli was characterized. The substrate specificity for L-arabinose was 166-fold higher than that for D-galactose. The optimal pH and temperature for the galactose isomerization reaction were 8.0 and 30 °C, respectively. The enzyme activity was stable for 1 h at temperatures below 35 °C and within a pH range of 8–10. The Michaelis constant, K m, for galactose was 1480 mM, which is 25-fold higher than that for arabinose. The addition of Fe2+ and Mn2+ ions enhanced the conversion of galactose to tagatose, whereas the addition of Cu2+, Zn2+, Hg2+, and Fe3+ ions inhibited the reaction completely. In the presence of 1 mM Fe2+ ions, the K m for galactose was found to be 300 mM.
Similar content being viewed by others
References
Banerjee, S., Anderson, F. & Farber, G.K. 1995 The evolution of sugar isomerases. Protein Engineering 8, 1189-1195.
Beadle, J.R., Saunder, J.P. & Wajada, T.J. 1992 Process for manufacturing tagatose. WO Patent 92/12263.
Buemann, B., Toubro, S. & Astrup, A. 1999 Human gastrointestinal tolerance to D-tagatose. Regulatory Toxicology and Pharmacology 29, S71-S77.
Cheetham, P.S.J. & Wootton, A.N. 1993 Bioconversion of D-galactose into D-tagatose. Enzyme and Microbial Technology 15, 105-108.
Dische, Z. & Borenfreund, E. 1951 A new spectrophotometric method for the detection and determination of keto sugar and trioses. Journal of Biological Chemistry 192, 583-587.
Englesberg, E., Irr, J., Power, J. & Lee, N. 1965 Positive control of enzyme synthesis by gene C in the L-arabinose system. Journal of Bacteriology 90, 946-957.
Izumori, K., Miyoshi, T., Tokuda, S. & Yamabe, K. 1984 Production of D-tagatose from ducitol by Arthrobacter globiformis. Applied and Environmental Microbiology 46, 1055-1057.
Izumori, K. & Tsuzaki, K. 1988 Production of D-tagatose from D-galactitol by Mycobacterium smegmatis. Journal of Fermentation Technology 66, 225-227.
Kim, S.Y., Roh, H.J. & Oh, D.K. 1997 D-tagatose production from D-galactose by Enterobacter agglomerans TY-25. Korean Journal of Applied Microbiology and Biotechnology 25, 490-494.
Kim, P., Roh, H.J., Yoon, S.H. & Choi, J.H. 1999 Recombinant strain of Escherichia coli harboring arabinose isomerase and tagatose production thereby. WO Patent pending PCT/KR99/00661.
Kim, P., Yoon, S.H., Roh, H.J. & Choi, J.H. 2001 High production of D-tagatose, a potential sugar substitute, using immobilized L-arabinose isomerase. Biotechnology Progress 17, 208-210.
Livesey, G. & Brown, J.C. 1996 D-tagatose is a bulk sweetener with zero energy determined in rats. Journal of Nutrition 126, 1601-1609.
Marzur, A.W. 1989 Functional sugar substitutes with reduced calories. European Patent 341,062.
Muniruzzanman, S., Tokunaga, H. & Izumori, K. 1994 Isolation of Enterobacter agglomerans strain 221e from soil, a potent D-tagatose producer from galactitol. Journal of Fermenation and Bioengineering 78, 145-148.
Nakamatu, T. & Yamanaka, K. 1969 Crystalization of L-arabinose isomerase from Lactobacillusgayonii. Biochimica et Biophysica Acta 178, 156-165.
Patrick, J.W. & Lee, N. 1968 Purification and properties of an L-arabinose isomerase from Escherichia coli. Journal of Biological Chemistry 243, 4312-4318.
Patrick, J. & Lee, N. 1975 L-arabinose isomerase. Methods in Enzymology 41, 453-458.
Roh, H.J., Kim, P., Park, Y.C. & Choi, J.H. 2000a Bioconversion of D-galactose into D-tagatose by expression of L-arabinose isomerase. Biotechnology and Applied Biochemistry 31, 1-4.
Roh, H.J., Yoon, S.H. & Kim, P. 2000b Preparation of L-arabinose isomerase originated from Escherichia coli as a biocatalyst for D-tagatose production. Biotechnology Letters 22, 197-199.
Zehener, L.R. 1988 D-tagatose as a low-calories carbohydrate sugar and bulking agent. European Patent 257,626.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Yoon, SH., Kim, P. & Oh, DK. Properties of L-arabinose isomerase from Escherichia coli as biocatalyst for tagatose production. World Journal of Microbiology and Biotechnology 19, 47–51 (2003). https://doi.org/10.1023/A:1022575601492
Issue Date:
DOI: https://doi.org/10.1023/A:1022575601492