Abstract
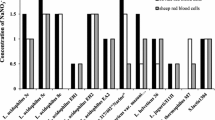
Milk oligosaccharides have been proposed to play an important role in newborn defense, blocking bacterial adhesion to the intestinal mucosa and preventing infections. Some studies have been performed on human milk oligosaccharides. Here we checked whether bovine milk oligosaccharides would achieve the same protective action against the most common calf enteric pathogens. Seven enterotoxigenic Escherichia coli strains, isolated from diarrheic calves, were selected. All strains managed to agglutinate horse erythrocytes, and we therefore used the inhibition of hemagglutination in the presence of oligosaccharides as an indicator of the union between oligosaccharide and bacterial adhesins. Oligosaccharides from different stages of bovine lactation and standard oligosaccharides were assayed. Midlactation milk, in particular that corresponding to the transition period, proved to be the most efficient at inhibiting hemagglutination. The standard oligosaccharides used pointed to the preference of several strains (K99-, F41-, and F17-fimbriated) for α2,6-linked sialic acid. By contrast, B23 fimbriae exhibited higher affinity for α2,3-sialylated isomers and B64 seemed to require N-acetylglucosamine for binding.
Our results suggest a general trend for milk oligosaccharides. Probably they participate in the protection of newborn mammals from pathogens.
Similar content being viewed by others
References
Soto GE, Hultgren SJ, Bacterial adhesins: Common themes and variations in architecture and assembly, J Bacteriol 181, 1059–71 (1999).
Edwards RA, Puente JL, Fimbrial expression in enteric bacteria: A critical step in intestinal pathogenesis, Trends Microbiol 6, 282–87 (1998).
Levine MM, Escherichia coli that cause diarrhea: Enterotoxigenic, enteropathogenic, enteroinvasive, enterohemorrhagic, and enteroadherent, J Infect Dis 155, 377–89 (1987).
Chan R, Acres SD, Costerton JW, Use of specific antibody to demonstrate glycocalix, K99 pili, and the spatial relationships of K99+ enterotoxigenic Escherichia coli in the ileum of colostrumfed calves, Infect Immun 37, 1170–80 (1982).
Girón JA, Ho ASY, Schoolnik GK, Characterization of fimbriae produced by enteropathogenic Escherichia coli, J Bacteriol 175, 7391–403 (1993).
Cravioto A, Tello A, Villafan H, Ruiz J, del Vedovo S, Neeser JR, Inhibition of localized adhesion of enteropathogenic Escherichia coli to HEp-2 cells by immunoglobulin and oligosaccharide fractions of human colostrum and breast milk, J Infect Dis 163, 1247–55 (1991).
Crane JK, Azar SS, Stam A, Newburg DS, Oligosaccharides from human milk block binding and activity of the Escherichia coli heat-stable enterotoxin (STa) in T84 intestinal cells, J Nutr 124, 2358–64 (1994).
Newburg DS, Human milk glycoconjugates that inhibit pathogens, Curr Med Chem 6, 117–27 (1999).
Newburg DS, Do the binding properties of oligosaccharides in milk protect human infants from gastrointestinal bacteria? J Nutr 127, 980S–4S (1997).
Veh RW, Michalski J-C, Corfield AP, Sander-Weber M, Gies D, Schauer R, New chromatographic system for the rapid analysis and preparation of colostrum sialyloligosaccharides, J Chromatogr 212, 313–22 (1981).
Saito T, Itoh T, Adachi S, Presence of two neutral disaccharides containing N-acetylhexosamine in bovine colostrum as free forms, Biochim Biophys Acta 801, 147–50 (1984).
Gopal PK, Gill HS, Oligosaccharides and glycoconjugates in bovine milk and colostrum, Br J Nutr 84, S69–S74 (2000).
Martín MJ, Martín-Sosa S, García-Pardo LA, Hueso P, Distribution of bovine milk sialoglycoconjugates during lactation, J Dairy Sci 84, 995–1000 (2001).
Parkkinen J, Finne J, Achtman M, Väisänen V, Korhonen TK, Escherichia coli strains binding neuraminyl α 2-3 galactosides, Biochem Biophys Res Comm 111, 456–61 (1983).
Blanco J, Blanco M, Garabal JI, González EA, Enterotoxins, colonization factors and serotypes of enterotoxigenic Escherichia coli from humans and animals, Microbiologia SEM 7, 57–72 (1991).
Guinée PAM, Veldkemp J, Jansen WH, Improved Minca medium for the detection of K99 antigen in calf enterotoxigenic strains of Escherichia coli, Infect Immun 15, 676–8 (1977).
Kobata A, Isolation of oligosaccharides from human milk, Methods in Enzymol 28, 262–71 (1972).
Chaplin MF, Kennedy JF, Carbohydrate Analysis: A Practical Approach (IRL Press, Oxford, 1986), p. 2.
Michalski JC, Isolation of glycans by HPLC. In Methods on Glycoconjugates, edited by Verbert A (Harwood Academic Publishers, Chur, 1995), pp. 67–77.
Lindahl M, Wadström T, K99 surface haemagglutinin of enterotoxigenic E. coli recognize terminal N-acetylgalactosamine and sialic acid residues of glycophorin and other complex glycoconjugates, Vet Microbiol 9, 249–57 (1984).
Smit H, Gaastra W, Kamerling JP, Vliegenthart JFG, de Graaf FK, Isolation and structural characterization of the equine erythrocyte receptor for enterotoxigenic Escherichia coliK99fimbrial adhesin, Infect Immun 46, 578–84 (1984).
Kunz C, Rudloff S, Hintelman A, Pohlentz G, Egge H, High-pH anion-exchange chromatography with pulsed amperometric detection and molar response factors of human milk oligosaccharides, J Chromatogr B 685, 211–21 (1996).
Newburg DS, Oligosaccharides and glycoconjugates in human milk: Their role in host defense, J Mamm Gland Biol Neopl 1, 271–83 (1996).
Andersson B, Porras O, Hanson LA, Lagergard T, Svanborg-Eden C, Inhibition of attachment of Streptococcus pneumoniae and Haemophilus influenzae by human milk and receptor oligosaccharides, J Infect Dis 153, 232–7 (1986).
Simon PM, Goode PL, Mobasseri A, Zopf D, Inhibition of Helicobacter pylori binding to gastrointestinal epithelial cells by sialic acid-containing oligosaccharides, Infect Immun 65, 750–7 (1997).
Engfer MB, Stahl B, Finke B, Sawatzki G, Daniel H, Human milk oligosaccharides are resistant to enzymatic hydrolysis in the upper gastrointestinal tract, Am J Clin Nutr 71, 1589–96 (2000).
Gnoth MJ, Kunz C, Kinne-Saffran E, Rudloff S, Human milk oligosaccharides are minimally digested in vitro, J Nutr 130, 3014–20 (2000).
Roy JHB, The Calf (Butterworths, London, 1988), p. 457.
Mouricout M-A, Julien RA, Pilus-mediated binding of bovine enterotoxigenic Escherichia coli to calf small intestinal mucins, Infect Immun 55, 1216–23 (1987).
Lindahl M, Wadström T, Binding to erythrocyte membrane glycoproteins and carbohydrate specificity of F41 fimbriae of enterotoxigenic Escherichia coli, FEMS Microbiol Lett 34, 297–300 (1986).
Mainil JG, Gérardin J, Jacquemin E, Identification of the F17 fimbrial subunit-and adhesin-encoding (f17A and f17G) gene variants in necrotoxigenic Escherichia coli from cattle, pigs and humans, Vet Microbiol 73, 327–35 (2000).
Bertin Y, Girardeau J-P, Darfeuille-Michaud A, Contrepois M, Characterization of 20K fimbriae, new adhesin of septicemic and diarrhea-associated Escherichia coli strains, that belongs to a family of adhesins with N-acetyl-D-glucosamine recognition, Infect Immun 64, 332–42 (1996).
Foley JA, Hunter AG, Otterby DE, Absorption of colostral proteins by newborn calves fed unfermented, fermented or buffered colostrum, J Dairy Sci 61, 1450–56 (1978).
BAMN Publication, A Guide to Dairy Calf Feeding and Management. Optimizing Rumen Development and Effective Weaning (Bovine Alliance Management and Nutrition Publications, Arlington, 1997).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Martín, MJ., Martín-Sosa, S. & Hueso, P. Binding of milk oligosaccharides by several enterotoxigenic Escherichia coli strains isolated from calves. Glycoconj J 19, 5–11 (2002). https://doi.org/10.1023/A:1022572628891
Issue Date:
DOI: https://doi.org/10.1023/A:1022572628891