Abstract
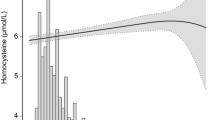
Background: Plasma total homocysteine (tHcy) level is an independent risk factor for cardiovascular disease (CVD) even among children. The purpose of this study is to evaluate the determinants and distributions of plasma tHcy levels and the relationship between plasma tHcy, folate and vitamin B12 levels among school children in Taipei. Methods: After multi-stage sampling, we randomly selected 1234 school children (609 boys and 625 girls) with the mean age of 13 years (from 12 to 15 years) in this study. Fasting plasma tHcy levels were measured using an ABBOTT IMx analyzer (Axis Biochemicals ASA, Oslo, Norway). Plasma folate and vitamin B12 levels were measured by ACS:180 automated chemiluminescence analyzer (Bayer, Tarrytown, NY, USA). Results: The distribution of plasma tHcy levels were skewed to the right with the mean values of 10.50 and 8.95 μmol/l and medians of 9.67 and 8.474 μmol/l for boys and girls, respectively. Plasma tHcy concentrations were lower in younger children and progressively increased with increasing age. Boys had significantly higher plasma tHcy levels than girls (10.50 ± 4.134 vs. 8.95 ± 2.61 μmol/l, p < 0.01) and lower plasma folate levels (6.05 ± 2.85 vs. 6.39 ± 2.58 nmol/l, p < 0.01), and vitamin B12 levels (444.8 ± 158.4 vs. 495.0 ± 181.5 pmol/l, p < 0.001). Plasma tHcy levels were significantly positively associated with anthropometric measures in boys; but these characteristics attenuated and became insignificant after adjusting for other potential confounders in girls. Plasma tHcy levels were negatively associated with plasma folate and vitamin B12 levels even after adjusting for BMI and other potential confounders in both genders. Conclusions: From this study, the distributions of tHcy levels were skewed to the right and the boys had higher plasma tHcy levels than girls. Plasma tHcy levels were significantly positively associated with BMI among boys. Further studies are needed to evaluate the relationship between tHcy and CVD risk factors among children for the better prevention of heart disease in early life.
Similar content being viewed by others
References
Finkelstein JD. Methionine metabolism in mammals. J NutrBiochem 1990; 1: 228–237.
Mudd SH, Levy HL, Skovby F. Disorders of transsulfuration. In: Scriver CG, Beudet AL, Sly WS, Valle D (eds), The Metabolic and MolecularBases of Inherited Disease, New York: McGraw Hill, 1995; 1279–1327.
Selhub J, Jacques PF, Wilson PW, Rush D, Rosenberg IH. Vitamin status and intake as primary determinants of homocysteinemia in an elderly population. JAMA 1993; 270: 2693–2698.
Guttormsen AB, Ueland PM, Nesthus I, et al. Determinants and vitamin responsiveness of intermediate hyperhomocysteinemia (> or = to 40 micro mol/liter): The Hordaland homocysteine study. J Clin Invest 1996; 98: 2174–2183.
Verhoef P, Stampfer MJ, Buring JE, et al. Homocysteine metabolism and risk of myocardial infarction: Relation with vitamins B6, B12 and folate. Am J Epidemiol 1996; 143: 845–859.
Ueland PM, Refsum H. Plasma homocysteine, a risk factorforvasculardisease: Plasma levels in health, disease, and drug therapy. J Lab Clin Med 1989; 114: 473–501.
Clarke R, Daly L, Robinson K, et al. Hyperhomocysteinemia: An independent risk factor for vascular disease. N Engl J Med 1991; 324: 1149–1155.
Malinow MR, Bostom AG, Krauss RM. Homocyst( e)ine, diet, and cardiovascular diseases: A statement for healthcare professionals from the Nutrition Committee. Circulation 1999; 99: 178–182.
Graham IM, Daly LE, Refsum HM, et al. Plasma homocysteine as a risk for vascular disease. JAMA 1997; 277: 1775–1781.
Andersson A, Brattstrom L, Israelsson B, Isaksson A, Hamfelt A, Hultberg B. Plasma homocysteine before and after methionine loading with regard to age, gender, and menopausal status. Eur J Clin Invest 1992; 22: 79–87.
Andersson A, Hultberg B, Brattstrom L, Isaksson A. Decreased serum homocysteine in pregnancy. Eur J Clin Chem Clin Biochem 1992; 30: 377–379.
Malinow MR. Homocyst(e)ine and arterial occlusive diseases. J Intern Med 1994; 236: 603–617.
Genest JJ, McNamara JR, Salem DN, Schaefer EJ. Prevalence of familial hyperhomocyst(e)inemia in men with premature coronary artery disease. Arterioscler Thromb 1991; 11: 1129–1136.
Brattstrom L, Lindgren A, Israelsson B, Andersson A, Hultberg B. Homocysteine and cysteine: Determinants of plasma levels in middle-aged and elderly subjects. J Intern Med 1994; 236: 633–641.
Refsum H, Wesenberg F, Ueland PM. Plasma homocysteine in children with acute lymphoblastic leukemia: Changes during a chemotherapeutic regimen including methotrexate. Cancer Res 1991; 51: 828–835.
Schneede J, Dagnelie PC, van Staveren WA, Vollset SE, Refsum H, Ueland PM. Methylmalonic acid and homocysteine in plasma as indicatorof functional cobalamin deficiency in infants on macrobiotic diets. PediatrRes 1994; 36: 194–201.
Tonstad S, Refsum H, Sivertsen M, Christophersen B, Ose L, Ueland PM. Relation of total homocysteine and lipid levels in children to premature cardiovascular death in male relatives. Pediatr Res 1996; 40: 47–52.
Tonstad S, Refsum H, Ueland PM. Association between total homocysteine and parental history of cardiovascular disease in children with familial hypercholesterolemia. Circulation 1997; 96: 1803–1808.
Ueland PM, Refsum H, Stabler SP, Malinow MR, Andersson A, Allen RH. Total homocysteine in plasma orser um: Methods and clinical application. Clin Chem 1993; 39: 1764–1779.
Vilaseca MA, Moyano D, Ferrer I, Artuch R. Total homocysteine in pediatric patients. Clin Chem 1997; 43: 690–692.
Reddy MN. Reference ranges for total homocysteine in children. Clin Chim Acta 1997; 262: 153–155.
De Laet C, Wautrecht JC, Brasseur D, et al. Plasma homocysteine concentrations in a Belgian school-age population. Am J Clin Nutr 1999; 69: 968–972.
Chu NF, Rimm EB, Wang DJ, Liou HS, Shieh SM. Relationship between anthropometric variable and lipid levels among school children: The Taipei Children Heart Study. Int J Obes 1998; 22: 66–72.
Chu NF, Rimm EB, Wang DJ, Liou HS, Shieh SM. Clustering of cardiovascular disease risk factors among obese schoolchildren: The Taipei Children Heart Study. Am J Clin Nutr 1998; 67: 1141–1146.
Shipchandler MT, Moore EG. Rapid fully automated measurement of plasma homocyst(e)ine with Abbott IMx® analyzer. Clin Chem 1995; 41: 991–994.
Blick K, Fry G, Tostenson G, Dunn D, Gillum R. Evaluation of automated folate, vitamin B12 and ferritin chemiluminescence assays on the Ciba Corning ACS:180 random-Access system. Clin Chem 1993; 39: 1215.
Malinow MR. Hyperhomocyst(e)inemia. A common and easily reversible risk factor for occlusive atherosclerosis. Circulation 1990; 81: 2004–2006.
Kang S-S, Wong PWK, Malinow MR. Hyperhomocyst( e)inemia as a risk factor for occlusive vascular disease. Annu Rev Nutr 1992; 12: 279–299.
Jacques PF, Sulsky SI, Sadowski JA, Phillips JC, Rush D, Willett WC. Comparison of micronutrient intake measured by a dietary questionnaire and biochemical indicators of micronutrient status. Am J Clin Nutr 1993; 57: 182–189.
Jacobsen DW. Homocysteine and vitamins in cardiovasculardisease. Clin Chem 1998; 44: 1833–1843.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Chang, JB., Chu, NF., Shen, MH. et al. Determinants and distributions of plasma total homocysteine concentrations among school children in Taiwan. Eur J Epidemiol 18, 33–38 (2003). https://doi.org/10.1023/A:1022504602101
Issue Date:
DOI: https://doi.org/10.1023/A:1022504602101