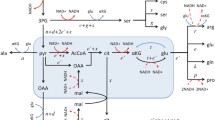
Abstract
Primary cultures of mitogen-activated rat thymocytes were used to study energy metabolism, gene expression of glycolytic enzymes, and production of reactive oxygen species during cell cycle progression. During transition from the resting to the proliferating state a 7- to 10-fold increase of glycolytic enzyme induction occurs which enables the cells to meet the enhanced energy demand by increased aerobic glycolysis. Cellular redox changes have been found to regulate gene expression of glycolytic enzymes by reversible oxidative inactivation of Spl-binding to the cognate DNA-binding sites in the promoter region. In contrast to nonproliferating cells, production of phorbol 12-myristate 13-acetate (PMA)-primed reactive oxygen species (ROS) in proliferating rat thymocytes and HL-60 cells is nearly abolished. Pyruvate, a product of aerobic glycolysis, is an effective scavenger of ROS, which could be shown to be generated mainly at the site of complex III of the mitochondrial respiratory chain. Aerobic glycolysis by proliferating cells is discussed as a means to minimize oxidative stress during the phases of the cell cycle when maximally enhanced biosynthesis and cell division do occur.
Similar content being viewed by others
REFERENCES
Aisenberg, A. C. (1961). The Glycolysis and Respiration of Tumors, Academic Press, London.
Ammendola, R., Mesuraca, M., Russo, T., and Cimino, F. (1994). Eur. J. Biochem. 225, 483–489.
Andrae, U., Singh, J., and Ziegler-Skylakakis, K. (1985). Toxicol. Lett. 28, 93–98.
Ardawi, M. S., and Newsholme, E. A. (1982). Biochem. J. 208, 743–748.
Argiles, J. M., and Lopez-Soriano, F. J. (1990). Med. Hypotheses 32, 151–155.
Arora, K. K., and Pedersen, P. L. (1988). J. Biol. Chem. 263, 17422–17428.
Ashizawa, K., Willingham, M. C., Liang, C. M., and Cheng, S. Y. (1991). J. Biol. Chem. 266, 16842–16846.
Baggetto, L. G. (1992). Biochimie 74, 959–974.
Balinsky, D., Platz, C. E., and Lewis, J. W. (1983). Cancer Res. 43, 5895–5901.
Barron, J. T., Kopp, S. J., Tow, J. P., and Parrillo, J. E. (1991). Biochim. Biophys. Acta 1093, 125–134.
Bartley, J. C., Barber, S., and Abraham, S. (1975). Cancer Res. 35, 1649–1653.
Baumann, M., Jezussek, D., Lang, T., Richter, R. T., and Brand, K. (1988). Tumour Biol. 9, 281–286.
Board, M., Humm, S., and Newsholme, E. A. (1990). Biochem. J. 265, 503–509.
Brand, K. (1985). Biochem. J. 228, 353–361.
Brand, K. (1987). J. Biol. Chem. 262, 15232–15235.
Brand, M. D. (1990). Biochim. Biophys. Acta 1018, 128–133.
Brand, K., and Hermfisse, U. (1997). FASEB J. 11, 388–395.
Brand, K., Leibold, W., Luppa, P., Schoerner, C., and Schulz, A. (1986). Immunobiology 173, 23–34.
Brand, K., Aichinger, S., Forster, S., Kupper, S., Neumann, B., Nürnberg, W., and Ohrisch, G. (1988). Eur. J. Biochem. 172, 695–702.
Burk, D., Woods, M., and Hunter, J. (1967). J. Natl. Cancer Inst. 38, 839–863.
Bustamante, E., and Pedersen, P. L. (1977). Proc. Natl. Acad. Sci. USA 74, 3735–3739.
Bustamante, E., Morris, H. P., and Pedersen, P. L. (1981). J. Biol. Chem. 256, 8699–8704.
Collins, S. J., Ruscetti, F. W., Gallagher, R. E., and Gallo, R. C. (1978). Proc. Natl. Acad. Sci. USA 75, 2458–2462.
Crabtree, H. G. (1929). Biochem. J. 23, 536–545.
Crepin, K. M., Darville, M. I., Hue, L., and Rousseau, G. G. (1989). Eur. J. Biochem. 183, 433–440.
Diaz-Espada, F., and Lopez-Alarcon, L. (1982). Immunology 46, 705–712.
Dröge, W., Roth, S., Altmann, A., and Mihm, S. (1987). Cell. Immunol. 108, 405–416.
Dunaway, G. A., and Karsten, T. P. (1985). J. Biol. Chem. 260, 4180–4185.
Eigenbrodt, E. and Glossmann, H. (1980). Trends Pharmacol. Sci. 1, 240–245.
Eigenbrodt, E., Gerbracht, U., Mazurek, S., Presek, P., and Friis, R. (1985). In Biochemical and Molecular Aspects of Selected Cancers, Vol. 2, Academic Press, New York, pp. 311–385.
Gilat, D., Hershkoviz, R., Goldkorn, I., Cahalon, L., Korner, G., Vlodavsky, I., and Lider, O. (1995). J. Exp. Med. 181, 1929–1934.
Goldstone, S. D., Fragonas, J. C., Jeitner, T. M., and Hunt, N. H. (1995). Biochim. Biophys. Acta 1263, 114–122.
Greiner, E. F., Guppy, M., and Brand, K. (1994). J. Biol. Chem. 269, 31484–31490.
Guppy, M., Greiner, E., and Brand, K. (1993). Eur. J. Biochem. 212, 95–99.
Guse, A. H., Greiner, E., Emmrich, F., and Brand, K. (1993). J. Biol. Chem. 268, 7129–7133.
Hamm-Künzelmann, B., Schäfer, D., Weigert, C., and Brand, K. (1997). FEBS Lett. 403, 87–90.
Hermfisse, U., Schäfer, D., Netzker, R., and Brand, K. (1996). Biochem. Biophys. Res. Commun. 225, 997–1005.
Hue, L., and Rider, M. H. (1987). Biochem. J. 245, 313–324.
Irani, K., Xia, Y., Zweier, J. L., Sollott, S. J., Der, C. J., Fearon, E. R., Sundaresan, M., Finkel, T., and Goldschmidt-Clermont, P. J. (1997). Science 275, 1649–1652.
Krebs, H. A. (1981). In Glutamine: Metabolism, Enzymology and Regulation (Mora, J., and Palacios, R., eds.), Academic Press, New York, pp. 319–329.
LaNoue, K. F., Hemington, J. G., Onishi, T., Morris, H. P., and Williamson, J. R. (1977). In Hormones and Cancer, Academic Press, New York, pp. 311–385.
Los, M., Schenk, H., Hexel, K., Baeuerle, P. A., Dröge, W., and Schulze-Osthoff, K. (1995). EMBO J. 14, 3731–3740.
Marjanovic, S., Wielburski, A., and Nelson, B. D. (1988). Biochim. Biophys. Acta 970, 1–6.
Marjanovic, S., Wollberg, P., Skog, S., Heiden, T., and Nelson, B. D. (1993). Arch. Biochem. Biophys. 302, 398–401.
Mazurek, S., Michel, A. and Eigenbrodt, E. (1997). J. Biol Chem. 272, 4941–4952.
McKeehan, W. L. (1982). Cell. Biol. Int. Rep. 6, 635–650.
Mujica, A., Moreno-Rodriguez, R., Naciff, J., Neri, L., and Tash, J. S. (1991). J. Reprod. Fertil. 92, 75–87.
Netzker, R., Greiner, E., Eigenbrodt, E., Noguchi, T., Tanaka, T., and Brand, K. (1992). J. Biol. Chem. 267, 6421–6424.
Netzker, R., Hermfisse, U., Wein, K. H., and Brand, K. (1994). Biochim. Biophys. Acta 1224, 371–376.
Netzker, R., Weigert, C., and Brand, K. (1997). Eur. J. Biochem. 245, 174–181.
Noguchi, T. Inoue, H., Nakamura, Y., Chen, H. L., Matsubara, K., and Tanaka, T. (1984). J. Biol. Chem. 259, 2651–2655.
O'Donnell-Tormey, J., Nathan, C. F., Lanks, K., DeBoer, C. J., and De La Harpe, J. (1987). J. Exp. Med. 165, 500–514.
Ouchi, M., and Ishibashi, S. (1975). Biochem. J. 149, 481–483.
Oude-Weernink, P. A., Rijksen, G., and Staal, G. E. (1991). Tumour Biol. 12, 339–352.
Pedersen, P. L. (1978). Prog. Exp. Tumor Res. 22, 190–274.
Poli, V., Mancini, F. P., and Cortese, R. (1990). Cell 63, 643–653.
Racker, E. (1976). J. Cell Physiol. 89, 697–700.
Reitzer, L. J., Wice, B. M., and Kennell, D. (1979). J. Biol. Chem. 254, 2669–2676.
Reitzer, L. J., Wice, B. M., and Kennell, D. (1980). J. Biol. Chem. 255, 5616–5626.
Rose, I. A., and Warms, V. B. (1967). J. Biol. Chem. 242, 1635–1645.
Schäfer, D., Hamm-Künzelmann, B., Hermfisse, U., and Brand, K. (1996). FEBS Lett. 391, 35–38.
Schöbitz, B., Netzker, R., Hannappel, E., and Brand, K. (1991). Eur. J. Biochem. 199, 257–262.
Schulze-Osthoff, K., Bakker, A. C., Vanhaesebroeck, B., Beyaert, R., Jacob, W. A., and Fiers, W. (1992). J. Biol. Chem. 267, 5317–5323.
Seshagiri, P. B., and Bavister, B. D. (1991). Mol. Reprod. Dev. 30, 105–111.
Tollefsbol, T. O., and Cohen, H. J. (1985). J. Cell Physiol. 123, 417–424.
Wang, T., Marquardt, C. and Foker, J. (1976). Nature 261, 702–705.
Warburg, O. (1929). Biochem. Z. 204, 482–483.
Warburg, O. (1930). The Metabolism of Tumors, Arnold Constable, London.
Warburg, O., Poesener, K., and Negelein, E. (1924). Biochem. Z. 152, 309–344.
Weber, G. (1977). N. Engl. J. Med. 296, 486–492 and 541–551.
Weber, G. and Morris, H. P. (1963). Cancer Res. 23, 987–994.
Weinhouse, S. (1966). Gann Monogr. 1, 99–115.
Weinhouse, S. (1976). Z. Krebsforsch. Klin, Onkol. 87, 115–126.
Zielke, H. R., Zielke, C. L., and Ozand, P. T. (1984). Fed. Proc. 43, 121–125.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Brand, K. Aerobic Glycolysis by Proliferating Cells: Protection against Oxidative Stress at the Expense of Energy Yield. J Bioenerg Biomembr 29, 355–364 (1997). https://doi.org/10.1023/A:1022498714522
Issue Date:
DOI: https://doi.org/10.1023/A:1022498714522