Abstract
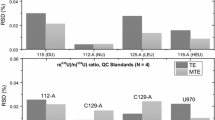
The experimental conditions for minimizing errors in the laser mass spectrometric (LMS) analysis of isotopic ratios for Nd and Yb were found, and the optimum isotope pairs for determining these elements with the use of isotope dilution (ID) were chosen. The precision and accuracy of LMS data, which are characterized by relative random errors (RSD) and relative systematic errors (δ), can be improved using ID. The values for Nd or Yb were no higher than RSD = 2.9% and δ = 0.024 or RSD = 5% and δ = 0.097, respectively.
Similar content being viewed by others
REFERENCES
De Bievre, P., Anal. Proc., 1993, no. 30, p. 328.
Becker, J.S. and Dietze, H.-J., Fresenius' J. Anal. Chem., 1999, vol. 364, no. 5, p. 482.
Inorganic Mass Spectrometry, Adams, F., Gijbels, R., and Van Grieken, R., Eds., New York: Wiley, 1988.
Jochum, K.P., Seufert, H.M., and Best, S., Fresenius'J.Anal. Chem., 1981, vol. 309, no. 4, p. 308.
Heumann, K.G., Gallus, S.M., Radlinger, G., and Vogl, J.,J. Anal. At. Spectrom., 1998, vol. 13, no. 9,p.1001.
Jochum, K.P., Laue, H.J., Seufert, H.M., et. al., Fresenius'J. Anal. Chem., 1997, vol. 359, nos. 4—5, p. 385.
Oksenoid, K.G., Ramendik, G.I., Sotnichenko, E.A., etal.,Zh. Anal. Khim., 1990, vol. 45, no. 6, p. 1197.
Heuman, K.G.,Fresenius' J. Anal. Chem., 1986, vol. 324, no. 6, p. 601.
Catterick, T., Fairman, B., and Harrington, C., J. Anal. At. Spectrom., 1998, vol. 13, no. 9, p. 1009.
Vanhaecke, F., Moens, L., and Dams, R., J. Anal. At. Spectrom., 1998, vol. 13, no. 10, p. 1189.
Maaskant, J.F.N., Boekholt, A.H., Jenks, P.J.,et al., Fresenius' J. Anal. Chem., 1998, vol. 360, nos. 3—4, p. 406.
De Bievre, P. and Peiser, H.S., Fresenius' J. Anal. Chem., 1997, vol. 359, nos. 7—8, p. 523.
Kinaeva, I.V., Ramendik, G.I., and Tyurin, D.A., Zh. Anal. Khim., 1991, vol. 46, no. 9, p. 1718.
Kinaeva, I.V., Ramendik, G.I., and Tyurin, D.A., Zh. Anal. Khim., 1992, vol. 47, no. 5, p. 820.
Ramendik, G.I., Kinaeva, I.V., and Tjurin, D.A., Fresenius' J. Anal. Chem., 1996, vol. 354, p. 150.
Ramendik, G.I., Sevast'yanov, V.S., and Fatyushina, E.V., Zh. Anal. Khim., 2000, vol. 55, no. 1, p. 13.
Alvarez, R., Paulsen, P.J., and Kelleher, D.E., Anal. Chem., 1969, vol. 41, no. 5, p. 955.
Analytical Methods for Atomic Spectrometry, Norwalk: Perkin-Elmer, 1976.
Stepanov, A.I., Ramendik, G.I., and Fatyushina, E.V., Zavod. Lab., 2001, vol. 67, no. 12, p. 7.
Isotopic Composition of the Elements (International Union of Pure and Applied Chemistry), Rosman, K.J.R. and Taylor, P.D.P., Eds., 1997, p. 18.
Ramendik, G.I., Sotnichenko, E.A., Oksenoid, K.G., et al., Zh. Anal. Khim., 1989, vol. 44, no. 8, p. 1361.
Kryuchkova, O.I., Ramendik, G.I., Khromov, A.Yu., and Chernoglazova, G.I., Zh. Anal. Khim., 1988, vol. 43, no.8, p. 1397.
Oksenoid, K.G., Ramendik, G.I., and Shuranova, N.G., Zh. Anal. Khim., 1995, vol. 50, no. 11, p. 1203.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ramendik, G.I., Fatyushina, E.V. & Stepanov, A.I. Determination of Neodymium and Ytterbium by Isotope Dilution Laser Mass Spectrometry. Journal of Analytical Chemistry 58, 152–155 (2003). https://doi.org/10.1023/A:1022358105429
Issue Date:
DOI: https://doi.org/10.1023/A:1022358105429