Abstract
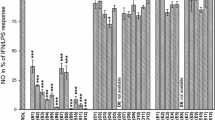
In order to further investigate the potential of rifamycins as antiinflammatory drugs, twenty-five semisynthetic rifamycins were tested at concentrations ranging from 10−9 to 10−5 M on in vitro human neutrophil functions such as locomotion, superoxide anion production, and degranulation, under different stimulatory conditions. They were also tested as antiproliferative agents on peripheral blood lymphocytes. The present semisynthetic derivatives are in general characterized by their carrying a hydrophilic substituent; they are rifamycin S or rifamycin SV derivatives carrying at C(3) either a carboxyalkyl side-chain or a glycosyl side-chain. Derivatives of the former group displayed inhibitory activities covering the whole range of activities tested, suggesting that the sum of these different effects could support their antiinflammatory activity in vivo. These derivatives, carrying a free carboxyl, are more water soluble than rifamycin SV at physiological pH, and may serve as antiinflammatory drugs for local administration, alternative to rifamycin SV, possibly giving higher efficacy and reduced side effects of pain and tissue swelling.
Similar content being viewed by others
REFERENCES
Brufani, M. 1977. In Topics in Antibiotic Chemistry (editor Sammes PC), 91–217. Ellis Horwood Ltd, Chichester.
Paunescu, E. 1970. In vivo and in vitro suppression of humoral and cellular immunological response by rifampicin. Nature 228:1188–1190.
Calleja, C., J. C. Pascussi, J. C. Mani, P. Maurel, and M. J. Vilarem. 1998. The antibiotic rifampicin is a nonsteroidal ligand and activator of the human glucocorticoid receptor. Nature Medicine 4:92–96.
Caruso, I., N. Principi, G. D'Urbino, S. Santandrea, L. Boccassini, F. Montrone, P. Puttini, A. Bombaci, and A. Bozzato. 1993. Rifamycin SV administered by intra-articular infiltrations shows disease modifying activity in patients with pauci-or polyarticular juvenile rheumatoid arthritis. J. Int. Med. Res. 21:243–256.
Caruso, I., S. Santandrea, P. Puttini, F. Montrone, L. Boccassini, V. Azzolini, M. Cazzola, and D. Dell'Acqua. 1992. Prevention of appearance of radiological lesions in early rheumatoid arthritis, a randomized single-blind study comparing intra-articular rifamycin with auranofin. J. Int. Med. Res. 1:61–77.
Arrigoni-Martelli, E., P. Schiatti, and D. Selva. 1971. Rifampicin and alanosine inhibition of adjuvant arthritis in rats. Pharm. Res. Commun. 3:239–245.
Marchesoni, A., L. Sinigaglia, R. Ranza, M. Varenna, N. Battafarano, and S. Tosi. 1993. Rifamycin SV versus triamcinolone in local treatment of rheumatoid synovitis. Scand. J. Rheumatol. 22:194–198.
Gabriel, S. E., D. L. Conn, and H. Luthra. 1990. Rifampicin therapy in rheumatoid arthritis. J. Rheumatol. 17:163–166.
Lindblad, S., E. Hedfors, and A. S. Malmborg. 1985. Rifamycin SV in local treatment of synovitis, a clinical, arthroscopic and pharmacological evaluation. J. Rheumatol. 12:900–903.
Di Giulio, A., A. Barracchini, L. Cellai, C. Martuccio, G. Amicosante, A. Oratore, G. C. Pantaleoni. 1996. Rifamycins as inhibitors of collagenase activity: their possible pharmacological role in collagen degradative diseases. Int. J. Pharm. 144:27–35.
Spisani, S., S. Traniello, C. Martuccio, O. Rizzuti, L. Cellai. 1997. Rifamycins inhibit human neutrophil functions: new derivatives with potential antiinflammatory activity. Inflammation 21:391–400.
Cellai, L., C. Martuccio, M. Marzano, A. Mochi Onori, L. Mannina, A. Benedetto, A. Di Caro, S. Lociuro, F. Ripamonti. 1997. Synthesis and activity studies on 3-(carboxyalkylthio) rifamycin S derivatives. Gazz. Chim. It. 127:317–323.
Bartolucci, C., Cellai, C. Martuccio, A. Rossi, A. Segre, S. Rizea-Savu, and L. Silvestro. 1996. Synthesis of glycosylrifamycins, a new type of semisynthetic rifamycins. Helv. Chim. Acta. 79:1611–1619.
Malech, H. L., and M. D. Gallin. 1987. Neutrophils in human diseases. N. Engl. J. Med. 317:687–694.
Zigmond, S. H. and J. G. Hirsch. 1973. Leukocyte locomotion and chemotaxis: new method for evaluation and demonstration of a derived chemotactic factor. J. Exp. Med. 137:387–410.
Gavioli, R., S. Spisani, A. L. Giuliani, E. Cosulich, A. Risso, and S. Traniello. 1991. CD16 and CR3 receptors distinguished between the two mechanisms of tumor cytotoxicity in neutrophils. British J. Haematol. 79:387–410.
Bergmeyer, H. U. 1983. Lactato Dehydrogenase. In Methods of enzymatic analysis, Vol. III, 118–133. Academic Press, London.
Biagi, G. L., A. M. Barbaro, M. F. Gamba, and M. C. Guerra. 1969. Partition data of penicillins determined by means of reversed-phase thin-layer chromatography. J. Chromatogr. 41:371–379.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Spisani, S., Traniello, S., Onori, A.M. et al. 3-(Carboxyalkylthio) Rifamycin S and SV Derivatives Inhibit Human Neutrophil Functions. Inflammation 22, 459–469 (1998). https://doi.org/10.1023/A:1022341909396
Issue Date:
DOI: https://doi.org/10.1023/A:1022341909396