Abstract
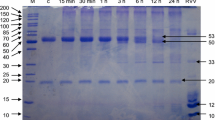
The binding of two matrix metalloproteinases (MMP) to fibrin was evaluated. MMP-2 (72-kDa) and MMP-9 (92-, 130-, and 225-kDa) were selected since both contain a fibronectin-like region and fibronectin binds fibrin. Gelatin zymography indicated selective and dose dependent binding of MMP-9 to fibrin. No MMP-2 binding to fibrin occurred. Densitometry revealed that the 130- and 225-kDa forms demonstrated similar sigmoidal binding profiles whereas 92-kDa uptake was hyperbolic. Fibronectin and TIMP-1 competition studies indicated that the fibronectin and C-terminal MMP-9 domains, respectively, were not involved with fibrin binding. The MMP-9 collagen-like region may be of regulatory significance since type I and II fibrillar and type IV basement membrane collagens demonstrated fibrin binding. During fibrinolysis, latent fibrin-bound MMP-9 was processed to lower molecular weight forms consistent with proteolytic activation. This process was inhibited by ∈-aminocaproic acid, indicating a plasmin-dependent pathway. The significance of these findings to procoagulant activity and MMP-mediated extracellular matrix destruction during inflammation and tumor invasion and metastasis is discussed.
Similar content being viewed by others
REFERENCES
Woessner, J. F., Jr. 1994. The family of matrix metalloproteinases. Ann. N. Y. Acad. Sci. 731:11–21.
Nagase, H. 1996. Matrix metalloproteinases. In: Zinc Metalloproteinases in Health and Disease, N. M. Hooper, ed. Taylor and Francis, London, England. pp. 153–204.
Stetler-Stevenson, W. G., S. Aznavoorian, and L. S. Liotta. 1993. Tumor cell interactions with the extracellular matrix during invasion and metastasis. Ann. Rev. Cell Biol. 9:541–573.
Cliffton, E. E., and D. Agostino. 1965. The effects of fibrin formation and alterations in the clotting mechanism on the development of metastases. Vasc. Dis. 2:43–52.
Dvorak, H. F. 1986. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. New Engl. J. Med. 315:1650–1659.
Wysocki, A. B., L. Staiano-Coico, and F. Grinnell. 1993. Wound fluid from chronic leg ulcers contains elevated levels of metalloproteinases MMP-2 and MMP-9. J. Invest. Dermatol. 101:64–68.
Mignatti, P., and D. B. Rifkin. 1996. Plasminogen activators and matrix metalloproteinases in angiogenesis. Enz. Prot. 49:117–137.
DeClerck, Y. A., and W. E. Laug. 1996. Cooperation between matrix metalloproteinases and the plasminogen activator-plasmin system in tumor progression. Enz. Prot. 49:72–84.
Rickles, F. R., and R. L. Edwards. 1983. Activation of blood coagulation in cancer: Trousseau's syndrome revisited. Blood 62:14–31.
Egyud, L. G., and B. Lipinski. 1991. Significance of fibrin formation and dissolution in the pathogenesis and treatment of cancer. Med. Hypoth. 36:336–340.
Tryggvason, K., M. Hoyhtya, and T. Salo. 1987. Proteolytic degradation of extracellular matrix in tumor invasion. Biochim. Biophys. Acta. 907:191–217.
Mignatti, P., and D. B. Rifkin. 1993. Biology and biochemistry of proteinases in tumor invasion. Physiol. Rev. 73:161–195.
Koolwijk, P. A., M. M. Miltenburg, M. G. M. van Erck, M. Oudshoorn, M. J. Niedbala, F. C. Breedveld, and V. W. M. van Hinsbergh. 1995. Activated gelatinase-B (MMP-9) and urokinase-type plasminogen activator in synovial fluids of patients with arthritis. Correlation with clinical and experimental variable of inflammation. J. Rheumatol. 22:385–393.
Brown, D. L., M. S. Hibbs, M. Kearney, C. Loushin, and J. M. Isner. 1995. Identification of 92-kDa gelatinase in human coronary atherosclerotic lesions. Circulation 91:2125–2131.
Van Wart, H. E., and H. Birkedal-Hansen. 1990. The cysteine switch: a principle of regulation of metalloproteinase activity with potential applicability to the entire matrix metalloproteinase gene family. Proc. Natl. Acad. Sci. U.S.A. 87:5578–5582.
Nagase, H. 1997. Activation mechanisms of matrix metalloproteinases. Biol. Chem. 378:151–160.
Mazzieri, R., L. Masiero, L. Zanetta, S. Monea, M. Onisto, S. Garbisa, and P. Mignatti. 1997. Control of type IV collagenase activity by components of the urokinase-plasmin system: a regulatory mechanism with cell-bound reactants. EMBO J. 16:2319–2332.
Ginestra, A., S. Monea, G. Seghezzi, V. Dolo, H. Nagase, P. Mignatti, and M. L. Vittorelli. 1997. Urokinase plasminogen activator and gelatinases are associated with membrane vesicles shed by human HT1080 fibrosarcoma cells. J. Biol. Chem. 272:17216–17222.
Rakoczi, I., B. Wiman, and D. Collen. 1978. On the biological significance of the specific interaction between fibrin, plasminogen and antiplasmin. Biochim. Biophys. Acta. 540:295–300.
Liotta, L. A., R. H. Goldfarb, R. Brundage, G. P. Siegal, V. Terranova, and S. Garbisa. 1981. Effect of plasminogen activator (urokinase), plasmin, and thrombin on glycoprotein and collagenous components of basement membrane. Cancer Res. 41:4629–4636.
Pepper, M. S., A. P. Sappino, R. Stocklin, R. Montesano, L. Orci, and J. D. Vassalli. 1993. Upregulation of urokinase receptor expression on migrating endothelial cells. J. Cell Biol. 122:673–684.
Hoylaerts, M., D. C. Rijken, H. R. Lijnen, and D. Collen. 1982. Kinetics of the activation of plasminogen by human tissue plasminogen activator. J. Biol. Chem. 257:2912–2919.
Blasi, F., M. P. Stoppelli, and M. V. Cubellis. 1986. The receptor for urokinase plasminogen activator. J. Cell Biochem. 32:179–186.
Blasi, F., J-D. Vassalli, and K. Dano. 1987. Urokinase-type plasminogen activator: proenzyme, receptor, and inhibitors. J. Cell Biol. 104:801–804.
Moscatelli, D., and D. B. Rifkin. 1988. Membrane and matrix localization of proteinases: a common theme in tumor cell invasion and angiogenesis. Biochim. Biophys. Acta 948:67–85.
Brooks, P. C., S. Stromblad, L. C. Sanders, T. L. von Schalscha, and R. T. Aimes. 1996. Localization of matrix metalloproteinase MMP-2 to the surface of invasive cells by interaction with the integrin αvβ3. Cell 85:683–693.
Monsky, W. L., T. Kelly, C. Y. Lin, Y. Yeh, W. G. Stetler-Stevenson, S. C. Mueller, and W. T. Chen. 1993. Binding and localization of M(r) 72,000 matrix metalloproteinase at cell surface invadopodia. Cancer Res. 53:3159–3164.
Murphy, G., F. Willenbock, R. V. Ward, M. I. Crockett, D. Eaton, and A. J. Docherty. 1992. The C-terminal domain of 72 kDa gelatinase A is not required for catalysis, but is essential for membrane activation and modulates interactions with tissue inhibitors of metalloproteinases. Biochem. J. 283:637–641.
Okada, Y., J. P. Bellocq, N. Rouyer, M. P. Chenard, M. C. Rio, P. Chambon, and P. Basset. 1995. Membrane-type metalloproteinase (MT-MMP) gene is expressed in stromal cells of human colon, breast, head and neck carcinomas. Proc. Natl. Acad. Sci. U.S.A. 92:2730–2734.
Sato, H., T. Takino, Y. Okada, J. Cao, A. Shinagawa, E. Yamamoto, and M. Seiki. 1994. A matrix metalloproteinase expressed on the surface of invasive tumor cells. Nature 370:61–65.
Stathakis, N. E., M. W. Mosesson, A. B. Chen, and D. K. Galanakis. 1978. Cryoprecipitation of fibrin-fibrinogen complexes induced by the cold-insoluble globulin of plasma. Blood 51:1211–1222.
Hibbs, M. S., K. A. Hasty, J. M. Seyer, A. H. Kang, and C. L. Mainardi. 1985. Biochemical and immunological characterization of the secreted forms of neutrophil gelatinase. J. Biol. Chem. 260:2493–2500.
Vartio, T., and M. Baumann. 1989. Human gelatinase/type IV procollagenase is a regular plasma component. FEBS Lett. 255:285–289.
Makowski, G. S., and M. L. Ramsby. 1996. Calibrating gelatin zymograms with human gelatinase standards. Anal. Biochem. 236:353–356.
Bodden, M. K., G. J. Harber, B. Birkedal-Hansen, L. J. Windsor, N. C. M. Caterina, J. A. Engler, and H. Birkedal-Hansen. 1994. Functional domains of human TIMP-1 (tissue inhibitor of metalloproteinases). J. Biol. Chem. 269:18943–18952.
Markert, M., P. C. Andrews, and B. M. Babior. 1984. Measurement of O −2 production by human neutrophils. The preparation and assay of NADPH oxidase-containing particles from human neutrophils. Meth. Enzymol. 105:358–365.
Clauss, A. 1957. Rapid physiological coagulation method for the determination of fibrinogen. Acta. Haematol. 17:237–246.
Regoeczi, E. 1968. Occlusion of plasma proteins by human fibrin: studies using trace-labelled proteins. Br. J. Haematol. 14:279–290.
Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685.
Heussen, C., and E. B. Dowdle. 1980. Electrophoretic analysis of plasminogen activators in polyacrylamide gels containing sodium dodecyl sulfate and copolymerized substrates. Anal. Biochem. 102:196–202.
Makowski, G. S., and M. L. Ramsby. 1993. pH modification to enhance the molecular seiving properties of sodium dodecyl sulfate-10% polyacrylamide gels. Anal. Biochem. 212:283–285.
Lucas, M. A., L. J. Fretto, and P. A. McKee. 1983. Binding of human plasminogen to fibrin and fibrinogen. J. Biol. Chem. 258:4249–4256.
Francis, C. W., V. J. Marder, and G. H. Barlow. 1980. Plasmic degradation of crosslinked fibrin. Characterization of new macromolecular soluble complexes and a model of their structure. J. Clin. Invest. 66:1033–1043.
Fearnley, G. R., and J. M. Tweed. 1952. Evidence of an active fibrinolytic enzyme in the plasma of normal people with observation on inhibition associated with the presence of calcium. Clin. Sci. 1953; 12:81–89.
Chakrabarti, R., M. Bielawiec, J. F. Evans, and G. R. Fearnley. 1968. Methodological study and a recommended technique for determining the euglobulin lysis time. J. Clin. Pathol. 21:698–701.
Goldberg, G. I., B. L. Marmer, G. A. Grant, A. Z. Eisen, S. Wilhelm, and C. He. 1989. Human 72-kilodalton type IV collagenase forms a complex with a tissue inhibitor of metalloprotease designated TIMP-2. Proc. Natl. Acad. Sci. U.S.A. 86:8207–8211.
Murphy, G., and T. Crabbe. 1995. Gelatinases A and B. Meth. Enzymol. 248:470–484.
Strongin, A., I. Collier, P. Krasnov, L. Teresa Genrich, B. Marmer, and G. Goldberg. 1993. Human 92 kDa type IV collagenase: functional analysis of fibronectin and carboxyl-end domains. Kidney Intl. 43:158–162.
Preissner, K. T. 1989. The role of vitronectin as multifunctional regulator in the hemostatic and immune systems. Blut 59:419–431.
Mirshahi, M., J. Soria, H. Lu, C. Soria, M. Samama, and J. P. Caen. 1988. Defective thrombolysis due to collagen incorporation in fibrin clots. Thromb. Res. S8:73–80.
Grinnell, F., M. Feld, and D. Minter. 1980. Fibroblast adhesion to fibrinogen and fibrin substrata: requirement for cold-insoluble globulin (plasma fibronectin). Cell 19:517–525.
Steffen, L. W., and B. W. Steffen. 1976. Improved method for measuring fibrinogen in plasma, with use of a plasmin inhibitor. Clin. Chem. 22:381–383.
Richardson, D. L., D. S. Pepper, and A. B. Kay. 1976. Chemotaxis for human monocytes by fibrinogen-derived peptides. Br. J. Haematol. 32:507–513.
Wachtfogel, Y. T., W. Abrams, U. Kucich, G. Weinbaum, M. Schapira, and R. W. Colman. 1988. Fibronectin degradation products containing the cytoadhesive tetrapeptide stimulate human neutrophil degranulation. J. Clin. Invest. 81:1310–1316.
Rowland, F. N., M. J. Donovan, P. T. Picciano, G. D. Wilner, and D. L. Kreutzer. 1984. Fibrin-mediated vascular injury: identification of fibrin peptides that mediate endothelial cell retraction. J. Pathol. 117:P418–428.
Ramsby, M. L., and D. L. Kreutzer. 1993. Fibrin induction of tissue plasminogen activator in corneal endothelial cells in vitro. Invest. Ophthalmol. Vis. Sci. 34:3207–3219.
Ramsby, M. L., and D. L. Kreutzer. 1993. Fibrin induction of thrombospondin in corneal endothelial cell in vitro. Invest. Ophthalmol. Vis. Sci. 34:165–174.
Ramsby, M. L., and D. L. Kreutzer. 1994. Fibrin induction of interleukin-8 (IL-8) in corneal endothelial cell in vitro. Invest. Ophthalmol. Vis. Sci. 35:3980–3990.
Salo, T., M. Makela, M. Kylmaniemi, H. Autio-Harmainen, and H. Larjava. 1994. Expression of matrix metalloproteinase-2 and-9 during early wound healing. Lab. Invest. 70:176–182.
Matsubara, M., M. T. Girard, C. L. Kublin, C. Cintron, and M. E. Fini. 1991. Differential roles for two gelatinolytic enzymes of the matrix metalloproteinase family in the remodelling cornea. Dev. Biol. 147:425–439.
Brown, L. F., A. M. Dvorak, and H. F. Dvorak. 1989. Leaky vessels, fibrin deposition, and fibrosis: a sequence of events common to solid tumors and many other types of disease. Am. Rev. Respir. Dis. 140:1104–1107.
Galis, Z. S., G. K. Sukova, M. W. Lark, and P. Libby. 1994. Increased expression of matrix metalloproteinases and matrix degrading activity in vulnerable regions of human atherosclerotic plaques. J. Clin. Invest. 94:2493–2503.
Young, P. K., and F. Grinnell. 1994. Metalloproteinase activation cascade after burn injury: a longitudinal analysis of the human wound environment. J. Invest. Dermatol. 103:660–664.
Collier, I. E., S. M. Wilhelm, A. Z. Eisen, B. L. Marmer, G. A. Grant, J. L. Seltzer, A. Kronberger, C. He, E. Bauer, and G. I. Goldberg. 1988. H-ras oncogene-transformed human bronchial epithelial cells (TBE-1) secrete a single metalloproteinase capable of degrading basement membrane collagen. J. Biol. Chem. 263:6579–6587.
Keski-Oja, J., J. Lohi, A. Tuuttila, K. Tryggvason, and T. Vartio. 1992. Proteolytic processing of the 72,000-Da type-IV collagenase by urokinase plasminogen activator. Exp. Cell Res. 202:471–476.
Morodomi, T., Y. Ogata, Y. Sasaguri, M. Morimatsu, and H. Nagase. 1992. Purification and characterization of matrix metalloproteinase-9 from U937 monocytic leukaemia and HT1080 fibrosarcoma cells. Biochem. J. 285:603–611.
Okada, Y., T. Morodomi, J. J. Enghild, K. Suzuki, A. Yasui, I. Nakanishi, G. Salvesen, and H. Nagase. 1990. Matrix metalloproteinase 2 from human rheumatoid synovial fibroblasts. Purification and activation of the precursor and enzymic properties. Eur. J. Biochem. 194:721–730.
Lijnen, H. R., and D. Collen. 1982. Interaction of plasminogen activators and inhibitors with plasminogen and fibrin. Sem. Thromb. Hemost. 8:2–10.
Murphy, G., S. Atkinson, R. Ward, J. Gavrilovic, and J. J. Reynold. 1992. The role of plasminogen activators in the regulation of connective tissue metallproteinases. Ann. N. Y. Acad. Sci. 667:1–12.
Dvorak, H. F., D. R. Senger, and A. M. Dvorak. 1983. Fibrin as a component of the tumor stroma: origins and biological significance. Cancer Metast. Rev. 2:41–73.
Dvorak, H. F., A. M. Dvorak, E. J. Manseau, L. Wiberg, and W. H. Churchill. 1979. Fibrin gel investment associated with line 1 and line 10 solid tumor growth, angiogenesis, and fibroplasia in guinea pigs. Role of cellular immunity, myofibroblasts, microvascular damage, and infarction in line 1 tumor regression. J. Natl. Cancer Inst. 62:1459–1472.
Nagy, J. A., L. F. Brown, D. R. Senger, N. Lanir, L. Van De Water, A. M. Dvorak, and H. F. Dvorak. 1988. Pathogenesis of tumor stroma generation: a critical role for leaky blood vessels and fibrin deposition. Biochim. Biophys. Acta. 948:305–326.
Cardinali, M., R. Uchino, and S. I. Chung. 1990. Interaction of fibrinogen with murine melanoma cells: covalent association with cell membranes and protection against recognition by lymophine-activated killer cells. Cancer Res. 50:8010–8016.
Fesus, L., and K. Laki. 1976. On coupling bovine fibrinogen to the surface of malignant murine plasma cells by means of transglutaminase. Biochem. Biophys. Res. Comm. 72:131–137.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Makowski, G.S., Ramsby, M.L. Binding of Latent Matrix Metalloproteinase 9 to Fibrin: Activation via a Plasmin-Dependent Pathway. Inflammation 22, 287–305 (1998). https://doi.org/10.1023/A:1022300216202
Issue Date:
DOI: https://doi.org/10.1023/A:1022300216202