Abstract
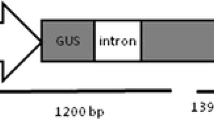
An efficient method for adventitious shoot regeneration for Arabis drummondii and a transformation protocol for A. gunnisoniana from hypocotyl explants are described. Hypocotyl explants from 7-day-old aseptically grown seedlings were cultured on MS medium containing plant growth regulators (6-benzylaminopurine, 1-phelyl-3- (1,2,3-thiadiazol-5-yl) urea, α-naphthaleneacetic acid and 2,4-dichlorophenoxy-acetic acid). After 4 weeks in culture, high frequency of adventitious shoot regeneration was observed. Regenerated shoots were rooted on half-strength MS basal medium supplemented 1% (w/v) sucrose, with or without NAA. This protocol was then used to produce transformed Arabis gunnisoniana plants. A. gunnisoniana hypocotyl explants were co-cultivated with Agrobacterium tumefaciens strain GV3101 harbouring pBJ40. Transgenic shoots were selected on MS 21 medium supplemented with 50 mg l kanamycin. PCR analysis verified the presence of the nptII gene in the plant DNA isolated from kanamycin resistant shoots.
Similar content being viewed by others
References
Asker S & Jerling L (1992) Apomixis in Plants. CRC Press, London
Barcaccia G, Mazzucado A, Albertini E, Zethof J, Gerats A, Pezzotti M & Falcinelli M (1998) Inheritance of parthenogenesis in Poa pratensis L.; auxin test and AFLP linkage analyses support monogenic control. Theor. Appl. Genet. 97: 74-82
Barghachi M, Turgut K, Scott R & Draper J (1994) High-frequency transformation from cultured cotyledons of Arabidopsis thaliana ecotypes C24 and Landsberg Erecta. Plant Growth Reg. 14: 61-67
Chaudhury AM, Ming L, Miller C, Craig S, Dennis ES & Peacock WJ (1997) Fertilization-independent seed development in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 94: 4223- 4228
Estopa M, Marfa V, Mele E & Messeguer J (2001) Study of different antibiotic combinations for use in the elimination of Agrobacterium with kanamycin selection in carnation. Plant Cell Tiss. Org. Cult. 65: 211-220
Grimanellii D, Leblanc O, Aspinosa E, Perotti E, Leon DG & Savidan V (1998) Mapping diplosporous apomixis in tetraploid Tripsacum; one gene or several genes? Heredity 80: 33-39
Khalafalla MM & Hattori K (1999) A combination of thidiazuron and bezyladenine promotes multiple shoot production from cotyledonary node explants of faba bean (Vicia faba). Plant Growth Reg. 27: 145-148
Koltunow AM, Bickneli RA & Chaudhury AM (1995) Apomixis: molecular strategies for the generation of genetically identical seeds without fertilization. Plant Physiol. 108: 1345-1352
Kuginuki Y & Tzukaaki H (2001) Regeneration ability and Agrobacterium-mediated transformation of different cultivars in Brassica oleracea L. and B. rapa L. (syn. B. campestris L.). J. Jpn. Soc. Hortic. Sci. 70: 682-690
Luo JP, Jia JF, Cu YG & Liu J (1999) High frequency somatic embryogenesis and plant regeneration in callus cultures of As-tragalus adsurgens Pall. Plant Sci. 143: 93-99
Matthews PR, Wang MB, Waterhouse PM, Thornton S, Fieg SJ, Gubler F & Jacobsen JV (2001) Marker gene elimination from transgenic barley, using co-transformation with adjacent ‘twin T-DNAs’ on a standard Agrobacterium transformation vector. Mol. Breed. 7: 195-202
Murashige T & Skoog F (1962) A revised medium for rapid growth and bioassays with tabacco tissue culture. Physiol. Plant. 15: 473-497
Nogler GA (1984) Gametophytic apomixis. In: Johri BM (ed) Embryology of Angiosperms (pp. 475-518). Springer-Verlag, Berlin
Ozcan S, Barghchi M, Firek S & Draper J (1992) High frequency adventitous shoot regeneration from immature cotyledons of peas (Pisum sativum L.). Plant Cell Rep. 11: 44-47
Ozias-Akins P, Lubbers EL, Hanna WW & McKay JW (1993) Transmission of the apomictic mode of reproduction in Pennisetum; co-inheritance of the trait and molecular markers. Theor. Appl. Genet. 85: 632-638
Roy B (1995) The breeding systems of six species of Arabis (Brassicaceae). Am. J. Bot. 82: 869-877
Tivarekar S & Eapen S (2001) High frequency plant regeneration from immature cotyledons of mungbean. Plant Cell Tiss. Org. Cult. 66: 227-230
Turgut K, Barghchi M & Scott R (1998) Efficient shoot regeneration and somatic embryogenesis from immature cotyledons of Brassica napus L. Plant Breeding 117: 503-504
Xie D & Hong Y (2001) In vitro regeneration of Acacia mangium via organogenesis. Plant Cell Tiss. Org. Cult. 66: 167-173
Victor JMR, Murch SJ, Krishna Ra\(\mathop {\text{j}}\limits^\prime \) & Saxena PK (1999) Somatic embryogenesis and organogenesis in peanut: the role of thidiazuron and N-benzylaminopurine in the induction of plant morphogenesis. Plant Growth Reg. 28: 9-15
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Taskin, K.M., Turgut, K., Gulhan Ercan, A. et al. Agrobacterium-mediated transformation of Arabis gunnisoniana . Plant Cell, Tissue and Organ Culture 72, 173–180 (2003). https://doi.org/10.1023/A:1022291324492
Issue Date:
DOI: https://doi.org/10.1023/A:1022291324492