Abstract
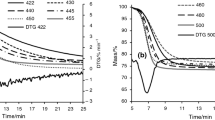
Organic matter evolution and kinetics of combustion of Tarfaya and Timahdit oil shales have been examined by thermogravimetry (TG) and by differential thermal analysis (DTA). An agreement is observed between both techniques where it was found that combustion of organic matter occurs in two steps. Kissinger's method applied on experimental results gives an activation energy of the same magnitude for the first step of both oil shales (103 kJ mol−1) whereas the second is 148 kJ mol−1 for Timahdit and 118 kJ mol−1 for Tarfaya.
The changes in specific surface area during thermal combustion of Timahdit and Tarfaya oil shales have been studied by thermogravimetric gas sorption balance and correlated with experimental results obtained on TG/DTA in air. For Timahdit oil shale oxidation products, specific surface areas calculated from nitrogen adsorption data shows a slight increase during the temperature domain of 280 to 430°C and after this temperature, they increase sharply. However, data obtained with Tarfaya oil shales shows a significant increase at the temperature of maximum oxidation of the first stage of combustion of organic matter.
Similar content being viewed by others
References
W. G. Schlinger and D. R. Jesse, I.&E.C. Proc. Des. Dev., 7 (1968) 275.
A. K. Burnham, Fuel, 58 (1978) 285.
I. C. Lee and H. Y. Sohn, Fuel, 65 (1986) 129.
C. M. Earnest, Thermochim. Acta, 60 (1983) 171.
J. H. Levy and W. I. Stuart, Thermochim. Acta, 74 (1984) 227.
A. Lamribah, L. Belkbir and S. A. A. Jayaweera, Qatar Univ. J., 14 (1994) 31.
J. P. Vantelon, C. Breillat, F. Gaboriaud and A. Alaoui-Sosse, Fuel, 69 (1990) 211.
K. Rajeshwar, R. Nottenburg and J. B. DuBow, J. Mater. Sci., 14 (1979) 2025.
K. Rajeshwar and J. B. DuBow, Fuel, 59 (1980) 790.
D. E. Rogers and D. M. Bibby, Thermochim. Acta, 30 (1979) 303.
H. R. Rose, D. R. Smith and A. M. Vassallo, Energy & Fuels, 12 (1998) 682.
L. Belkbir, H. Barkia and N. Gerard, Thermochim. Acta, 103 (1986) 147.
S. Brunauer, P. H. Emmett and E. Teller, J. Amer. Chem. Soc., 60 (1938) 309.
P. R. Tisot, J. Chem. Eng. Data, 7 (1962) 405.
C. A. Slettevold, A. H. Biermann and A. K. Burnham, L. L. N. L, Report UCRL-52619, Livermore, CA, 1978.
J. T. Schrodt and A. Ocampo, Fuel, 63 (1984) 1523.
O. M. Dogan and B. Z. Uysal, Fuel, 75 (1996) 1424.
H. Barkia, Doctorat d'état, Mohammed V-Agdal University, Faculty of Sciences, Rabat, Morocco, 1990.
H. Barkia, L. Belkbir and S. A. A. Jayaweera, Thermochim. Acta, 103 (1986) 147.
D. R. Glasson, J. Chem. Soc., 1956, pp. 1506-10.
H. E. Kissinger, Anal. Chem., 29 (1957) 1702.
A. K. Burnham and R. L. Braun, Energy & Fuels, 13 (1999) 1.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Barkia, H., Belkbir, L. & Jayaweera, S.A.A. Oxidation kinetics of timahdit and tarfaya moroccan oil shales. Journal of Thermal Analysis and Calorimetry 71, 97–106 (2003). https://doi.org/10.1023/A:1022262116440
Issue Date:
DOI: https://doi.org/10.1023/A:1022262116440