Abstract
For more than thirty years behaviour of phase transitions in solid oxygen has been the subject of an interest to thermometry because oxygen is one of few substances having three phase transitions in the low temperature range. The triple point of oxygen is the primary fixed point of the International Temperature Scale of 1990 and β – γ and α – β phase transitions are recommended as secondary fixed points.
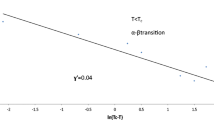
In this paper heat capacity of oxygen in the region of the α – β transition is investigated. The heat capacity peak, which has the total width of about 50 mK, has been observed to be composed, in reality, of two sharper peaks separeted by 20 mK. Some preliminary results of the β – γ transition investigation are also reported.
Similar content being viewed by others
REFERENCES
P. W. Stephens and C. F. Majkrzak, Phys. Rev. B 33, 1 (1986).
A. Jezowski, P. Stachowiak, V. V. Sumarokov, J. Mucha, and Yu. A. Freiman, Phys. Rev. Let. 71, 97 (1993).
I. I. Krupskii, A. K. Prokhvatilov, Yu. A. Freiman, and A. J. Erienburg, Fiz. Nizk. Temp. 5, 271 (1979) [Sov. J. Low Temp. Phys. 5, 130 (1979)].
M. P. Orlova, Temperature, Its Measurement and Control in Sciences and Industry vol. 3., Part 1, 179 (1962).
C. H. Fagerstroem and A. C. Hollins-Hallet, J. Low Temp. Phys. 1, 3–12 (1969).
J. Ancsin, in: Temperature Measurement 1975, ed. by B. F. Filling and T. J. Quinn, IOP Conf. Ser. No. 26, Inst. of Physics, Bristol (1975) p. 57.
W. F. Giauque and H. L. Johnston, J. Am. Chem. Soc. 51, 2300 (1929).
C. S. Barrett and L. Meyer, Phys. Rev. 160, 694–697 (1967).
H. M. Roder, J. Phys. Chem. Ref. Data 7 (3) 1 (1978).
R. LeSar, and R. D. Etters, Phys. Rev. B 37, 5364 (1988).
B. Kuchta, T. Luty, and R. J. Meier, J. Phys. C 20, 585 (1987).
H. J. Hoge, Rev. Natl. Bur. Std. 44, 321 (1950).
R. Muijwijk, Physica 43, 475 (1969).
H. Preston-Thomas, Metrologia 27, 3 (1990).
J. A. Cowan, R. C. Kemp, and W. R. G. Kemp, Metrologia 12, 87 (1976).
F. Pavese and G. Molinar, Modern Gas-Based Temperature and Pressure Measurements, Plenum, New York & London (1992).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Szmyrka-Grzebyk, A., Lipiński, L. & Manuszkiewicz, H. Phase Transitions in Solid Oxygen as Thermometric Fixed Points. Journal of Low Temperature Physics 111, 399–406 (1998). https://doi.org/10.1023/A:1022248006071
Issue Date:
DOI: https://doi.org/10.1023/A:1022248006071