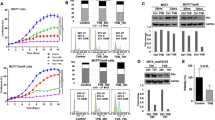
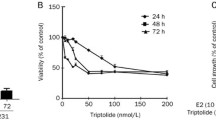
Abstract
Farnesyl transferase inhibitors (FTIs) serve to specifically inhibit farnesyl isoprenoid lipid modification of proteins. Although originally developed as anti-Ras oncoprotein drugs, it now appears that these compounds function independently of Ras. FTIs have been shown to inhibit transformation by a variety of mechanisms, including apoptosis involving cytochrome c release from mitochondria.
Tamoxifen exhibits both anti-estrogenic and estrogenic properties and is widely used as an estrogen antagonist for the treatment of estrogen receptor (ER) positive human breast tumors. Tamoxifen can induce ER-dependent apoptosis in human breast tumor cells by a mechanism involving the Bcl2/mitochondrial arm of the apoptotic machinery. Since tamoxifen and FTIs may stimulate distinct components of the mitochondrial-based apoptotic machinery, we reasoned that their effects might be synergistic.
Here we show that anti-estrogens and an FTI (FTI-277) synergize to inhibit cell growth and enhance cell death in ER positive, human breast tumor cell lines. However, the drugs exhibited only additive effects on an ER negative cell line. Analysis of treated ER positive T-47D cells demonstrated that a synergistic increase in apoptosis was induced, as measured by increased caspase 3 activity. Thus, tamoxifen and FTIs may synergize to promote apoptotic cell death in ER positive human breast tumor cells.
Similar content being viewed by others
References
Bos JL: Ras oncogenes in human cancer: a review. Cancer Res 49: 4682-4689, 1989
Clark GJ, Der CJ: Oncogenic activation of Ras proteins. In: Dickey BF, Birnbaumer L (eds) GTPases in Biology. Springer-Verlag, Berlin, 1993, pp. 259-287
Shirasawa S, Furuse M, Yokoyama N, Sasazuki T: Altered growth of human colon cancer cell lines disrupted at activated Ki-ras. Science 260: 85-88, 1993
Plattner R, Anderson MJ, Sato KY, Fasching CL, Der CJ, Stanbridge EJ: Loss of oncogenic ras expression does not correlate with loss of tumorigenicity in human cells. Proc Natl Acad Sci USA 93: 6665-6670, 1996
Miyakis S, Sourvinos G, Spandidos DA: Differential expression and mutation of the ras family genes in human breast cancer. Biochem Biophys Res Commun 251: 609-612, 1998
Clark GJ, Der CJ: Aberrant function of the Ras signal transduction pathway in human breast cancer. Breast Cancer Res Treat 35: 133-144, 1995
Gibbs JB, Oliff A, Kohl NE: Farnesyltransferase inhibitors: Ras research yields a potential cancer therapeutic. Cell 77: 175-178, 1994
Cox AD, Der CJ: Farnesyltransferase inhibitors and cancer treatment: targeting simply Ras? Biochim Biophys Acta 1333: F51-F71, 1997
Kato K, Cox AD, Hisaka MM, Graham SM, Buss JE, Der CJ: Isoprenoid addition to Ras protein is the critical modification for its membrane association and transforming activity. Proc Natl Acad Sci USA 89: 6403-6407, 1992
Magee AI, Newman CM, Giannakouros T, Hancock JF, Fawell E, Armstrong J: Lipid modifications and function of the ras superfamily of proteins. Biochem Soc Trans 20: 497-499, 1992
Sepp-Lorenzino L, Ma Z, Rands E, Kohl NE, Gibbs JB, Oliff A, Rosen N: A peptidomimetic inhibitor of farnesyl-protein transferase blocks the anchorage-dependent and-independent growth of human tumor cell lines. Cancer Res 55: 5302-5309, 1995
Ura H, Obara T, Shudo R, Itoh A, Tanno S, Fujii T, Nishino N, Kohgo Y: Selective cytotoxicity of farnesylamine to pancreatic carcinoma cells and Ki-ras-transformed fibroblasts. Mol Carcinog 21: 93-99, 1998
Kohl NE, Mosser SD, deSolms SJ, Giuliani EA, Pompliano DL, Graham SL, Smith RL, Scolnick EM, Oliff A, Gibbs JB: Selective inhibition of ras-dependent transformation by a farnesyltransferase inhibitor. Science 260: 1934-1937, 1993
James GL, Goldstein JL, Brown MS, Rawson TE, Somers TC, McDowell RS, Crowley CW, Lucas BK, Levinson AD, Marsters JC: Benzodiazepine peptidomimetics: potent inhibitors of Ras farnesylation in animal cells. Science 260: 1937-1942, 1993
Sun J, Qian Y, Hamilton AD, Sebti SM: Ras CAAX peptidomimetic FTI 276 selectively blocks tumor growth in nude mice of a human lung carcinoma with K-Ras mutation and p53 deletion. Cancer Res 55: 4243-4247, 1995
Trempus CS, Bishop WR, Njoroge FG, Doll RJ, Battalora MS, Mahler JF, Haseman JK, Tennant RW: A farnesyl transferase inhibitor suppresses TPA-mediated skin tumor development without altering hyperplasia in the ras transgenic Tg.AC mouse. Mol Carcinog 27: 24-33, 2000
Norgaard P, Law B, Joseph H, Page DL, Shyr Y, Mays D, Pietenpol JA, Kohl NE, Oliff A, Coffey RJ, Poulsen HS, Moses HL: Treatment with farnesyl-protein transferase inhibitor induces regression of mammary tumors in transforming growth factor (TGF) alpha and TGF alpha/neu transgenic mice by inhibition of mitogenic activity and induction of apoptosis. Clin Cancer Res 5: 35-42, 1999
Kohl NE, Omer CA, Conner MW, Anthony NJ, Davide JP, deSolms SJ, Giuliani EA, Gomez RP, Graham SL, Hamilton K: Inhibition of farnesyltransferase induces regression of mammary and salivary carcinomas in ras transgenic mice. Nat Med 1: 792-797, 1995
Liu A, Du W, Liu JP, Jessell TM, Prendergast GC: RhoB alteration is necessary for apoptotic and antineoplastic responses to farnesyltransferase inhibitors. Mol Cell Biol 20: 6105-6113, 2000
Andela VB, Rosenblatt JD, Schwarz EM, Puzas EJ, O'Keefe RJ, Rosier RN: Synergism of aminobisphosphonates and farnesyl transferase inhibitors on tumor metastasis. Clin Orthop JID-0075674 228-239, 2002
Adjei AA, Davis JN, Bruzek LM, Erlichman C, Kaufmann SH: Synergy of the protein farnesyltransferase inhibitor SCH66336 and cisplatin in human cancer cell lines. Clin Cancer Res JID-9502500 7: 1438-1445, 2001
Hoover RR, Mahon FX, Melo JV, Daley GQ: Overcoming STI571 resistance with the farnesyl transferase inhibitor SCH66336. Blood JID-7603509 100: 1068-1071, 2002
Cox AD, Der CJ: Farnesyltransferase inhibitors: promises and realities. Curr Opin Pharmacol JID-100966133 2: 388-393, 2002
Wakeling AE: Tissue-specific actions of antioestrogens. Mutat Res 333: 45-49, 1995
Candi E, De Melino GLV, Piacentini M, Guerrieri P, Spinedi A, Knight RA: Tamoxifen and somatostatin affect tumours by inducing apoptosis. Cancer Lett 96: 141-145, 1995
Wang TT, Phang JM: Effects of estrogen on apoptotic pathways in human breast cancer cell line MCF-7. Cancer Res 55: 2487-2489, 1995
Fattman CL, An B, Sussman L, Dou QP: p53-independent dephosphorylation and cleavage of retinoblastoma protein during tamoxifen-induced apoptosis in human breast carcinoma cells. Cancer Lett 130: 103-113, 1998
Ellis PA, Saccani-Jotti G, Clarke R, Johnston SR, Anderson E, Howell A, A'Hern R, Salter J, Detre S, Nicholson R, Robertson J, Smith IE, Dowsett M: Induction of apoptosis by tamoxifen and ICI 182780 in primary breast cancer. Int J Cancer 72: 608-613, 1997
Suzuki N, Urano J, Tamanoi F: Farnesyltransferase inhibitors induce cytochrome c release and caspase 3 activation preferentially in transformed cells. Proc Natl Acad Sci USA 95: 15356-15361, 1998
MacGregor JI, Jordan VC: Basic guide to the mechanisms of antiestrogen action. Pharmacol Rev 50: 151-196, 1998
deFazio A, Chiew YE, Sini RL, Janes PW, Sutherland RL: Expression of c-erbB receptors, heregulin and oestrogen receptor in human breast cell lines. Int J Cancer 87: 487-498, 2000
Cox AD, Der CJ: Protein prenylation: more than just glue? Curr Opin Cell Biol 4: 1008-1016, 1992
Lerner EC, Qian Y, Blaskovich MA, Fossum RD, Vogt A, Sun J, Cox AD, Der CJ, Hamilton AD, Sebti SM: Ras CAAX peptidomimetic FTI-277 selectively blocks oncogenic Ras signaling by inducing cytoplasmic accumulation of inactive Ras-Raf complexes. J Biol Chem 270: 26802-26806, 1995
Lippman M, Bolan G, Huff K: The effects of estrogens and antiestrogens on hormone-responsive human breast cancer in long-term tissue culture. Cancer Res 36: 4595-4601, 1976
Murphy LC, Dotzlaw H: Endogenous growth factor expression in T-47D, human breast cancer cells, associated with reduced sensitivity to antiproliferative effects of progestins and antiestrogens. Cancer Res 49: 599-604, 1989
Lee TH, Chuang LY, Hung WC: Induction of p21WAF1 expression via Sp1-binding sites by tamoxifen in estrogen receptor-negative lung cancer cells. Oncogene 19: 3766-3773, 2000
Clarke R, Leonessa F, Welch JN, Skaar TC: Cellular and molecular pharmacology of antiestrogen action and resistance. Pharmacol Rev 53: 25-72, 2001
Howell A, Osborne CK, Morris C, Wakeling AE: ICI 182,780 (Faslodex): development of a novel, ‘pure’ antiestrogen. Cancer 89: 817-825, 2000
Budihardjo I, Oliver H, Lutter M, Luo X, Wang X: Biochemical pathways of caspase activation during apoptosis. Annu Rev Cell Dev Biol 15: 269-290, 1999
Lebowitz PF, Prendergast GC: Non-Ras targets of farnesyltransferase inhibitors: focus on Rho. Oncogene 17: 1439-1445, 1998
Lerner EC, Zhang TT, Knowles DB, Qian Y, Hamilton AD, Sebti SM: Inhibition of the prenylation of K-Ras, but not Hor N-Ras, is highly resistant to CAAX peptidomimetics and requires both a farnesyltransferase and a geranylgeranyltransferase I inhibitor in human tumor cell lines. Oncogene 15: 1283-1288, 1997
Whyte DB, Kirschmeier P, Hockenberry TN, Nunez-Oliva I, James L, Catino JJ, Bishop WR, Pai JK: K-and N-Ras are geranylgeranylated in cells treated with farnesyl protein transferase inhibitors. J Biol Chem 272: 14459-14464, 1997
Prendergast GC, Davide JP, deSolms SJ, Giuliani EA, Graham SL, Gibbs JB, Oliff A, Kohl NE: Farnesyltransferase inhibition causes morphological reversion of ras-transformed cells by a complex mechanism that involves regulation of the actin cytoskeleton. Mol Cell Biol 14: 4193-4202, 1994
Forget MA, Desrosiers RR, Del M, Moumdjian R, Shedid D, Berthelet F, Beliveau R: The expression of rho proteins decreases with human brain tumor progression: potential tumor markers. Clin Exp Metastasis JID-8409970 19: 9-15, 1902
Adnane J, Muro-Cacho C, Mathews L, Sebti SM, Munoz-Antonia T: Suppression of rho B expression in invasive carcinoma from head and neck cancer patients. Clin Cancer Res JID-9502500 8: 2225-2232, 2002
Ashar HR, James L, Gray K, Carr D, Black S, Armstrong L, Bishop WR, Kirschmeier P: Farnesyl transferase inhibitors block the farnesylation of CENP-E and CENP-F and alter the association of CENP-E with the microtubules. J Biol Chem 275: 30451-30457, 2000
Zhang GJ, Kimijima I, Onda M, Kanno M, Sato H, Watanabe T, Tsuchiya A, Abe R, Takenoshita S: Tamoxifen-induced apoptosis in breast cancer cells relates to down-regulation of bcl-2, but not bax and bcl-X(L), without alteration of p53 protein levels. Clin Cancer Res 5: 2971-2977, 1999
Jiang K, Coppola D, Crespo NC, Nicosia SV, Hamilton AD, Sebti SM, Cheng JQ: The phosphoinositide 3-OH kinase/AKT2 pathway as a critical target for farnesyltransferase inhibitor-induced apoptosis. Mol Cell Biol 20: 139-148, 2000
Altan N, Chen Y, Schindler M, Simon SM: Tamoxifen inhibits acidification in cells independent of the estrogen receptor. Proc Natl Acad Sci USA 96: 4432-4437, 1999
Miquel K, Pradines A, Sun J, Qian Y, Hamilton AD, Sebti SM, Favre G: GGTI-298 induces G0-G1 block and apoptosis whereas FTI-277 causes G2-M enrichment in A549 cells. Cancer Res 57: 1846-1850, 1997
Zujewski J, Horak ID, Bol CJ, Woestenborghs R, Bowden C, End DW, Piotrovsky VK, Chiao J, Belly RT, Todd A, Kopp WC, Kohler DR, Chow C, Noone M, Hakim FT, Larkin G, Gress RE, Nussenblatt RB, Kremer AB, Cowan KH: Phase I and pharmacokinetic study of farnesyl protein transferase inhibitor R115777 in advanced cancer. J Clin Oncol 18: 927-941, 2000
Adjei AA, Erlichman C, Davis JN, Cutler DL, Sloan JA, Marks RS, Hanson LJ, Svingen PA, Atherton P, Bishop WR, Kirschmeier P, Kaufmann SH: A Phase I trial of the farnesyl transferase inhibitor SCH66336: evidence for biological and clinical activity. Cancer Res 60: 1871-1877, 2000
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ellis, C.A., Vos, M.D., Wickline, M. et al. Tamoxifen and the Farnesyl Transferase Inhibitor FTI-277 Synergize to Inhibit Growth in Estrogen Receptor-Positive Breast Tumor Cell Lines. Breast Cancer Res Treat 78, 59–67 (2003). https://doi.org/10.1023/A:1022105511409
Issue Date:
DOI: https://doi.org/10.1023/A:1022105511409