Abstract
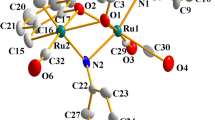
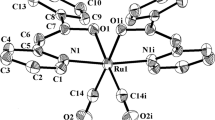
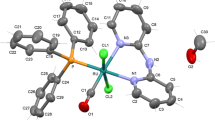
The oxidative addition reaction of 2,6-bis(bromomethyl)pyridine to Ru3(CO)12 gave scarcely soluble {Ru2Br2(μ-Q)(CO)4} n , 1, [Q=C5H3N-2-C(O)CH2-6-CH2] or a mixture of 1 and the mononuclear complex RuBr(Q′)(CO)3, 2, [Q′=C5H3N-2-C(O)CH2-6-CH2Br] according to the reactant's mole ratio. Further reactions of 1 with some N- and P-donor ligands (L) afforded readily soluble dinuclear complexes, Ru2(μ-Br)(μ-Q)Br(CO) n (L) m [n=4, m=1, L=PPh3 3a, or py 3b; n=3, m=2, L=PPh3 5a, or PPh2(o-tolyl) 5b]. In this paper, the characterization of these products by the elemental analyses and the spectroscopic methods are described. The X-ray crystal structures of Ru2(μ-Br) (μ-Q)Br(CO)4(PPh3)(MeOH), 4, which was obtained by crystallization of 3a from MeOH, and of 5a · (2CHCl 3 ) are also described. Each of the metal atoms in 4 has a distorted octahedral coordination, while in 5a · (2CHCl 3 ) one metal atom takes a distorted octahedral geometry and the other pseudooctahedral, which is completed by presenting a Ru ··· Br secondary bonding interaction.
Similar content being viewed by others
REFERENCES
K. Higashimura and Y. Nakamura (1993). J. Chem. Soc., Dalton Trans. 3075.
Y. Nakamura and N. Shinkawa (1994). Rhodium Ex. 6, 14; N. Shinkawa, A. Sato, J. Shinya, Y. Nakamura, and S. Okeya (1995). Bull. Chem. Soc. Jpn. 68, 183.
K. Yamasaki, H. Saito, M. Tadokoro, K. Matsumoto, S. Miyajima, and Y. Nakamura (1997). Bull. Chem. Soc. Jpn. 70, 2155.
E. Sappa, in E. W. Abel, F. G. A. Stone, and G. Wilkinson (eds.), Comprehensive Organometallic Chemistry, Vol. 7 (Pergamon, Oxford, 1995), Chap. 14, pp. 803–834; M. I. Bruce, M. P. Cifuentes, and M. G. Humphrey (1991). Polyhedron 10, 277.
M. Shimizu, Y. Nakamura, and M. Tadokoro (1996). Chem. Lett. 773; M. Shimizu, H. Saito, M. Tadokoro, and Y. Nakamura (1998). J. Chem. Soc., Dalton Trans. 51.
A. K. Rappe and C. J. Casewit, Calleo NMR, A Second-Order Nuclear Magnetic Resonance Spectral Simulation Application (Calleo Scientific Software Publishers, Fort Collins, CO, 1989), Chap. 1, p. 5.
M. Shimizu, Y. Nakamura, and M. Tadokoro (1997). Polyhedron 16, 577.
A. G. Orpen, L. Brammer, F. H. Allen, O. Kennard, D. G. Watson, and R. Taylor, in A. J. C. Wilson (ed.), International Tables for Crystallography, Vol. C (Kluwer Academic, Dordrecht, The Netherlands, 1992), Chap. 9, p. 774.
M. F. Richardson, G. Wulfsberg, R. Marlow, S. Zaghonni, D. Mccorkle, K. Shadid, J. Gagliardi, Jr., and B. Farris (1993). Inorg. Chem. 32, 1913.
N. W. Alcock (1972). Adv. Inorg. Radiochem. 15, 1.
R. J. Kulawiec and R. H. Crabtree (1990). Coord. Chem. Rev. 99, 69.
G. M. Sheldrick, SHELXS 86, Program for Crystal Structure Determination (University of Göttingen, 1986).
teXsan, Structure Analysis Package (Molecular Structure Corporation, Houston, Texas, 1985/1992).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Saito, H., Inoue, K., Matsumoto, K. et al. Dinuclear Ruthenium(II) Carbonyl Complexes Doubly Bridged by a Bromine Atom and a C5H3N-2-C(O)CH2-6-CH2 (Q) Group. Crystal Structures of Ru2(μ-Br)(μ-Q)Br(CO)4(PPh3)(MeOH) and Ru2(μ-Br)(μ-Q)Br(CO)3(PPh3)2 . Journal of Cluster Science 9, 145–164 (1998). https://doi.org/10.1023/A:1021990016044
Issue Date:
DOI: https://doi.org/10.1023/A:1021990016044