Abstract
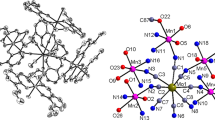
A new complex, [Sm(DMF)4(H2O)4Fe(CN)6]·H2O (DMF = N, N-dimethylformamide), has been synthesized and characterized by X-ray single crystal structure and thermogravimetric analyses. The complex crystallizes in the P21/n space group, with lattice parameters a = 17.583(4) Å, b = 8.870(2) Å, c = 19.845(6) Å, β = 95.98(3)°, V = 3078(1) Å3, D x = 1.679 Mg m−3, D m = 1.65(1) Mg m−3, Z = 4. The molecular structure shows that a cyano-bridged bimetallic structure is obtained. The Sm atom is coordinated by eight oxygen atoms of four water molecules and four DMF molecules and one nitrogen atom of the bridging cyanide ligand. The iron atom assumes approximately an octahedral environment surrounded by six CN ligands. The hydrate water molecule is hydrogen-bonded to one of the O atoms bound to Sm. Each terminal CN ligand of the Fe(CN) 3−6 entity is hydrogen-bonded to some O atoms of water molecules. An infrared spectrum is also reported.
Similar content being viewed by others
References
Behrens, U.; Brimah, A.K.; Soliman, T.M.; Fischer, R.D.; Apperley, D.C.; Davies, N.A.; Harris, R.K., Organometallics 1992, 11, 1719.
Lu, J.; Harrison, W.T.A.; Jacobson, A.J., Angew. Chem. Int. Ed. Engl. 1995, 34, 2557.
Brandt, P.; Brimah, A.K.; Fischer, R.D., Angew. Chem. Int. Ed. Engl. 1988, 27, 1521.
Eller, S.; Adam, M.; Fischer, R.D., Angew. Chem. Int. Ed. Engl. 1990, 29, 1126.
Brimah, A.K.; Siebel, E.; Fischer, R.D.; Davies, N.A.; Apperley, D.A.; Harris, R.K., J. Organomet. Chem. 1994, 475, 85.
Lu, J.; Harrison, W.T.A.; Jacobson, A.J., Inorg. Chem. 1996, 35, 4271.
Ohba, M.; Maruone, N.; Okawa, H.; Enoki, T.; Latour, J.-M., J. Am. Chem. Soc. 1994, 116, 11566.
Miyasaka, H.; Matsumoto, N.; Okawa, H.; Re, N.; Gallo, E.; Floriani, C., J. Am. Chem. Soc. 1996, 118, 981.
Miyasaka, H.; Matsumoto, N.; Re, N.; Gallo, E.; Floriani, C., Inorg. Chem. 1997, 36, 670.
Ohba, M.; Okawa, H.; Fukita, N.; Hashimoto, Y., J. Am. Chem. Soc. 1997, 119, 1011.
Ferlay, S.; Mallah, T.; Ouahes, R.; Vellet, P.; Verdaguer, M., Nature 1995, 378, 701.
Entley, W.R.; Girolami, G.S., Science 1995, 268, 397.
Michaut, C.; Ouahab, L.; Bergerat, P.; Kahn, O.; Bousseksou, A., J. Am. Chem. Soc. 1996, 118, 3610.
Sato, O.; Iyoda, T.; Fujishima, A.; Hashimoto, K., Science 1996, 272, 704.
Morpurgo, G.O.; Mosini, V.; Porta, P.; Dessy, G.; Fares, V., J. Chem. Soc. Dalton Trans. 1980, 1272.
Traversa, E.; Nunziante, P.; Sakamoto, M.; Watanabe, K.; Sadaoka, Y.; Sakai, Y., Chem. Lett. 1995, 189.
Sadaska, Y.; Traversa, E.; Sakamoto, M., Chem. Lett. 1996, 1747.
Knoeppel, D.W.; Shore, S.G., Inorg. Chem. 1996, 35, 1747.
Hulliger, F.; Landolt, M.; Vetsch, H., J. Solid State Chem. 1976, 18, 283.
Mikkigan, W.O.; Uda, M.; Dillin, D.R.; Bailey, W.E.; Williams, R.J., In Research and Develop. Progr. No. 723, Int-Osw-RDPR-71, 723, US Dep. of the Interior (Oct. 1971).
Knoeppel, D.W.; Shore, S.G., Inorg. Chem. 1996, 35, 1747, and references therein. Mullica, D.F.; Hayward, P.K.; Sappenfield. E.L., Inorg. Chim. Acta 1996, 253, 97. Knoeppel, D.W.; Shore, S.G., Inorg. Chem. 1996, 35, 5328.
Gilmore, G.J. MITHRIL, a computer program for the automatic solution of crystal structures from X-ray data; University of Glasgow: Scotland, 1983.
Ibers, J.A. International Tables for X-Ray Crystallography, Vol. IV, Kynoch Press: Birmingham, England, 1974.
Enraf-Nonius, CAD4 Operation Manual; Delft Instruments X-ray Diffraction PO BOX 811, 2600 AV Delft: The Netherlands, 1985, Version 5.0.
TEXSAN, Molecular Structure Corporation, Molecular Structure Corporation: MSC,3200A Research Forest Drive, The Woodlands, TX, 1987.
Johnson, C.K. ORTEPII, Report ORNL-5138; Oak Ridge National Laboratory: TN.
Vannerberg, N.-G., Acta Chem. Scand. 1972, 26, 2963.
Ouahab, L.; Bouherour, S.; Auffredic, J.-P.; Grandjean, D., J. Solid State Chem. 1989, 82, 139.
Brown, I.D. Acta Crystallogr. Sect. A 1976, 32, 24.
Mullica, D.F.; Tippin, D.B.; Sappenfield, E.L., J. Crystallogr. Spectrosc. Res. 1991, 21, 81.
Mullica, D.F.; Hayward, P.K.; Sappenfield, E.L., Inorg. Chim. Acta 1995, 237, 111.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kou, HZ., Yang, GM., Liao, DZ. et al. A dinuclear cyano-bridged complex based on hexacyanoferrate(III) and samarium(III) nitrate: synthesis and crystal structure. Journal of Chemical Crystallography 28, 303–307 (1998). https://doi.org/10.1023/A:1021857420915
Issue Date:
DOI: https://doi.org/10.1023/A:1021857420915