Abstract
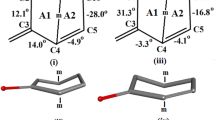
The title compound (C19H26N2O2) is one of a series of novel heteroatom steroidal derivatives recently prepared for testing their antifertility profiles and progesterone binding affinity. It is one of a pair of epimers differing in configuration at position 5. The X-ray analysis has uniquivocally resolved this ambiguity. The compound crystallizes in space group P212121, a = 9.257(3), b = 9.419(3), c = 19.089(5)Å, and Z = 4 and the structure was solved by direct methods. In the steroid skeleton Ring A does not exhibit the chair conformation commonly found in the steroid nucleus, being considerably strained, presumably as a consequence of the fused planar oxadiazole ring E. Rings B and C, however, are chairs and ring D is in an intermediate envelope/half-chair conformation. All rings of the steroid skeleton are trans connected.
Similar content being viewed by others
References
Singh, H.; Yadav, M.R.; Paul, D. Ind. J. Chem. 1985, 24B, 1158.
Clinton, R.O.; Manson, A.J.; Stonner, F.W.; Neumann, H.C.; Christiansen, R.G.; Clarke, R.L.; Ackerman, J.H.; Dean J.W.; Dickinson, W.B.; Carabates, C. J. Am. Chem. Soc. 1961, 83, 1478.
Shimizu, M.; Ohta, G.; Ueno. K.; Takegoshi, T.; Oshina, Y.; Kasahara, A.; Onodena, T.; Mogi, M.; Tackegawa, H. Chem. Pharm. Bull. 1965, 13, 895.
Kasahara, A.; Onodera, T.; Mogi, M.; Oshima, Y; Shimizu, M. Chem. Pharm. Bull. 1965, 13, 1460.
Kasahara, A.; Onodera, T.; Mogi, M.; Oshima, Y.; Shimizu, M. Chem. Pharm. Bull. 1966, 14, 285.
Havranek, R.E.; Hoey, G.B.; Baeder, D.H. J. Med. Chem. 1966, 9, 326.
Ohta, G.; Takeoshi, T.; Ueno, K.; Shimizu, M. Chem. Pharm Bull. 1965, 13, 1445.
Enraf-Nonius. CAD-4 Software. Enraf-Nonius: Delft, Holland, 1988.
Sheldrick, G.M. SHELX86: Program for the solution of crystal structures. University of Göttingen, Germany, 1986.
Sheldrick G.M. SHELX93: Program for the refinement of crystal structures; University of Göttingen: Germany, 1993.
Karaulov, A. SNOOPI: Molecular Plotting Program; University of Wales: Cardiff, Wales, 1992.
El Shora, A.; Palmer, R.A.; Singh, H.; Paul, D. J. Crystallogr. Spec. Res. 1984, 14, 315.
Duax, W.L.; Norton, D.A. Atlas of Steroid Structure; Plenum: New York, 1977.
Allen, F.H.; Kennard, O.; Watson, D.G.; Brannen, L.; Orpen, X.; Taylor, R. J. Chem. Soc. Perkin Trans. 1987, 2, S1.
Campsteyn, H.; Dupont, L.; Dideberge, O. Acta Crystallogr. 1972, B28, 3032.
Flack, H.D. Acta Crystallogr. 1983, A39, 876.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lisgarten, D.R., Palmer, R.A. Crystal structure of 17-oxo-5α-androstano [3,4-C] 1′2′5′-oxadiazole (HS1000). Journal of Chemical Crystallography 28, 725–729 (1998). https://doi.org/10.1023/A:1021856309954
Issue Date:
DOI: https://doi.org/10.1023/A:1021856309954