Abstract
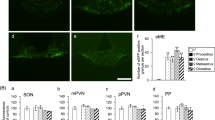
We studied the effect of endothelins (ETs) on receptor-mediated NO/cGMP signaling in rat arcuate nucleus–median eminence (AN-ME) fragments, an hypothalamic structure known to contain a rich plexus of nitric oxide synthase (NOS)-containing neurons and fibers together with densely arranged ETB-receptor-like immunoreactive fibers. NOS activity was determined measuring the conversion of [3H] arginine to [3H] citrulline, as an index of NO produced. cGMP production was determined by radio immunoassay. ET-1, ET-3, and the selective ETB receptor agonist, IRL1620, significantly increased cGMP formation and NOS activity. Preincubation of AN-ME fragment with L-arginine analog, N-nitro-L-arginine (L-NAME), inhibited ET-1 or IRL1620-stimulated cGMP formation. The addition of the selective ETB receptor antagonist, BQ788, blocked ET-1-, ET-3-, or IRL1620-induced increase in NOS activity and cGMP generation, while BQ123, a selective ETA receptor antagonist, was ineffective. Our results demonstrate that in whole rat AN-ME fragments, ETs stimulate NO/cGMP signaling pathway through the interaction with the ETB receptor subtype, supporting the concept that ETs may represent an important regulator of reproductive and neuroendocrine function.
Similar content being viewed by others
REFERENCES
Arai, H., Hori, S., Aramori, I., Ohkubo, H., and Nakanishi, S. (1990). Cloning and expression of a cDNA encoding an endothelin receptor. Nature 348:730–732.
Brann, D. W., Bhat, G. K., Lamar, C. A., and Mahesh, V. B. (1997). Gaseous transmitters and neuroendocrine regulation. Neuroendocrinology 65:385–395.
Bredt, D. S., Glatt, C. E., Hwang, P. M., Fotuhi, M., Dawson, T. M., and Snyder, S. H. (1991). Nitric oxide synthase protein and mRNAare discretely localized in neuronal populations of the mammalian CNS together with NADPH diaphorase. Neuron 7:615–624.
Bredt, D. S., and Snyder, S.H. (1990). Isolation of nitric oxide synthetase, a calmodulin-requiring enzyme. Proc. Natl. Acad. Sci. U.S.A. 87: 682–685.
Calogero, A. E., Raiti, F., Nicolosi, G., Burrello, N., D'Agata, R. D., and Matero, F. (1994). Effects of endothelin-1 and endothelin-3 on rat hypothalamic corticothophin-releasing hormone and pituitary ACTH release in vitro. J. Endocrinol. 140:419–424.
Costa, A., Trainer, R., Besser, M., and Grossman, A. (1993). Nitric oxide modulates the release of corticotropin-releasing hormone from the rat hypothalamus in vitro. Brain Res. 605:187–192.
D'Amico, M., Di Fillipo, C., and Rossi, F. (1998). Depresor response to endothelin into the superior colliculus of rats: Predominant role of endothelin ETB receptor. Eur. J. Pharmacol. 347:71–75.
De Vente, J., Hopkins, D. A., Markerink-Van Ittersum, M., Emson, P. C., Schmidt, H. H. H. W., and Steinbusch, H. W. M. (1998). Distribution of nitric oxide synthase and nitric oxide-receptive cyclic GMP-producing structures in the rat brain. Neuroscience 87:207–241.
Dinerman, L., Dawson, T. M., Schell, M. J., Snowman, A., and Snyder, S. H. (1994). Endothelial nitric oxide synthase localized to hippocampal pyramidal cells: Implications for synaptic plasticity. Proc. Natl. Acad. Sci. U.S.A. 91:4214–4218.
Di Nuncio, A. S., Jaureguiberry, M. S., Rodano, V., Bianciotti, L. G., and Vatta, M. S. (2002). Endothelin-1 and-3 diminish neuronal NE release through an NO mechanism in rat anterior hypothalamus. Am. J. Physiol. Regul. Integr. Comp. Physiol. 283:R615–R622.
Edwards, R. M., Pullen, M., and Nambi, P. (1992). Activation of endothelin ETB receptors increases glomerular cGMP via an L-arginine-dependent pathway. Am. J. Physiol. 263:F1020–F1025.
Evans, J. J. (1999). Modulation of gonadotrophin levels by peptides acting at the anterior pituitary gland. Endocrinol. Rev. 20:46–67.
Fujitani, Y., Udea, H., Okada, T., Urade, Y., and Karaki, H. (1993). A selective agonist of endothelin type B receptor, IRL1620, stimulates cyclic GMP increase via nitric oxide formation in rat aorta. J. Pharmacol. Exp. Ther. 267:683–689.
Giaid, A., Gibson, S. J., Herrero, M. T., Gentleman, S., Legon, S., Yanagisawa, M., Masaki, T., Ibrahim, N. B. N., Roberts, G.W., Rossi, M. L., and Polak, J. M. (1991). Topographical localisation of endothelin mRNA and peptide immunoreactivity in neurones of the human brain. Histochemistry 95:303–314.
Hagiwara, H., Nagasawa, T., Yamamoto, T., Lodhi, K. M., Ito, T., Takemura, N., and Hirose, S. (1993). Immunochemical characterization and localization of endothelin ETB receptor. Am. J. Physiol. 264:R777–R783.
Herbison, A. E., Simonian, S. X., Norris, P. J., and Emson, P. C. (1996). Relationship of neuronal nitric oxide synthase immunoreactivity to GnRH neurons in the ovariectomized and intact female rat. J. Neuroendocrinol. 8:73–82.
Hirata, Y., Emori, T., Eguchi, S., Kanno, K., Imai, T., Ohta, K., and Marumo, F. (1993). Endothelin receptor subtype B mediates synthesis of nitric oxide by cultured bovine endothelial cells. J. Clin. Invest. 91:1367–1373.
Hori, S.,Komatsu,Y., Shigemoto, R., Mizuno, M., and Nakanishi, S. (1992). Distinct tissue distribution and cellular localization of two messenger ribonucleic acids encoding different subtypes of rat endothelin receptors. Endocrinology 130:1885–1895.
Ishii, K., Warner, T. D., Sheng, H., and Murad, F. (1991). Endothelin increases cyclic GMP levels in LLCPK1 porcine kidney epithelial cells via formation of an endothelium-derived relaxing factor-like substance. J. Pharmacol. Exp. Ther. 259:1102–1108.
Israel, A., Garrido, M. R., Mathison, Y., Barbella, Y., and Becemberg, I. (1990). Brain Natriuretic Peptide stimulates particulate guanylate cyclase activity in selected areas of the rat brain. Neurosci. Lett. 114(1)114:107–112.
Kadekaro, M., Terrell, M. L., Liu, H., Gestl, S., Bui, V., and Summy-Long, J. Y. (1998). Effect of L-NAME on cerebral metabolic, vasopressin, oxytocin, and blood pressure responses in haemorrhaged rats. Am. J. Physiol. 274:R1070–R1077.
Kawakami, S., Ichikawa, M., Yokosuka, M., Tsukamura, H., and Maeda, K. (1998). Glial and neuronal localization of neuronal nitric oxide synthase immunoreactivity in the median eminence of female rats. Brain Res. 789:322–326.
Kawano, Y., Yoshida, K., Yoshimi, H., Kuramochi, M., and Omae, T. (1989). The cardiovascular effect of intracerebroventricular endothelin in rats. J. Hypertens. 7(Suppl.):S22–S23.
Knowles, R. G., Palacios, M., Palmer, R. M. J., and Moncada, S. (1989). Formation of nitric oxide from L-arginine in the central nervous system: A transduction mechanism for stimualtion of the soluble guanylate cyclase. Proc. Natl. Acad. Sci. U.S.A. 86:5159–5162.
Kohzuki, M., Chai, S. Y., Paxinos, G., Karavas, A., Casley D. J., Johnston, C. I., and Mendelshon, F. A. O. (1991). Localization and characterization of endothelin binding sites in the rat brain visualized by in vitro autoradiography. Neuroscience 42:245–260.
Kurokawa, K., Yamada, H., Liu, Y., and Kudo, M. (2000). Immunohistochemical distribution of the endothelin-converting enzyme-1 in the rat hypothalamo-pituitary axis. Neurosci. Lett. 284(1/2):81–84.
Kurokawa, K., Yamada, H., and Ochi, J. (1997). Topographical distribution of neurons containing endothelin type A receptor in the rat brain. J. Comp. Neurol. 389:348–360.
Lopez, F. J., Moretto, M., Merchenthaler, I., and Negro-Vilar, A. (1997). Nitric oxide is involved in the genesis of pulsatile LHRH secretion from immortalized LHRH neurons. J. Neuroendocrinol. 9:647–654.
Lowry, O., Rosebrough, N., Farr, A., and Randall, R. (1951). Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193:265–275.
Mathison, Y., and Israel, A. (1998). Endothelin ETB receptor subtype mediates nitric/oxide cGMP formation in rat adrenal medulla. Brain Res. Bull. 45:15–19.
McCumber, M.W., Ross, C. A., and Snyder, S.H. (1990). Endothelin in brain: Receptors, mitogenesis and biosynthesis in glial cells. Proc. Natl. Acad. Sci. U.S.A. 87:2358–2363.
Moretto, M., Lopez, F.J., and Negro-Vilar, A. (1993a) Endothelin-3 stimulates luteinizing hormonereleasing hormone (LHRH) secretion from LHRH neurons by a prostaglandin-dependent mechanism. Endocrinology 132:789–794.
Moretto, M., Lopez, F. J., and Negro-Vilar, A. (1993b). Nitric oxide regulates luteinizing hormone-releasing hormone secretion. Endocrinology 133:2399–2402.
Moritoki, H., Miyano, H., Takeuchi, S., Yamaguchi, M., Hisayama,T., and Kondoch,W. (1993). Endothelin-3-induced relaxation of rat thoracic aorta: A role for nitric oxide formation. Br. J. Pharmacol. 108:1125–1130.
Mosqueda-García, R., Fernandez-Violante, R., Hamakibo, T., and Stainback R. (1996). Vasopressin mediates the pressor effects of endothelin in the subfornical organ of the rat. J. Pharmacol. Exper. Ther. 277:1034–1042.
Mosqueda-García, R., Inagami, T., Appalsemy, M., Sugiura, M., and Robertson, R. M. (1993). Endothelin as a neuropeptide cardiovascular effects in the brainstem of normotensive rats. Circ. Res. 72:20–35.
Mosqueda-García, R., Yates, K., O'Leary, J., and Inagami, T. (1995). Cardiovascular and respiratory effect of endothelin in the ventrolateral medulla of normotensive rat. Hypertension 26:263–271.
Owada, A., Tomita, K., Terada, Y., Sakamoto, H., Nonoguchi, H., and Marumo, F. (1994). Endothelin (ET)-3 stimulates cyclic guanosine 30, 50-monophosphate production via ETB receptor by producing nitric oxide in isolated rat glomerulus, and in cultured rat mesangial cells. J. Clin. Invest. 93:556–563.
Prevot,V., Bouret, S., Stefabo, G. B., and Beauvillan, J.-C. (2000). Median eminence nitric oxide signalling. Brain Res. Rev. 34:21–41.
Rettori, V., Belova, N., Dees, W. L., Nyberg, C. L., Gimeno, M., and McCann, S. M. (1993). Role of nitric oxide in the control of luteinizing hormone-releasing hormone release in vivo and in vitro. Proc. Natl. Acad. Sci. U.S.A. 90:10130–10134.
Sakurai, T., Yanagisawa, M., Takura,Y., Miyazaki, H., Kimura, S., Goto, K., and Masaki, T. (1990). Cloning of a cDNAencoding a non-isopeptide-selective subtype of the endothelin receptor. Nature (London) 348:732–735.
Samson, W. K., Skala, K. D., Alexander, B. D., and Huang, F. L. S. (1991a). Possible neuroendocrine actions of endothelin-3. Endocrinology 128:1465–1473.
Samson, W. K., Skala, K. D., Alexander, B. D., and Huang, F. L. S. (1991b). Hypothalamic endothelin: Presence and effects related to fluid and electrolyte homeostasis. J. Cardiovasc. Pharmacol. 17:S346–S349.
Schuman, E. M., and Madison, D. V. (1994). Nitric oxide and synaptic function. Annu. Rev. Neurosci. 17:153–183.
Seidel, B., Stanarius, A., and Wolf, G. (1997). Differential expression of neuronal and endothelial nitric oxide synthase in blood vessels of the rat brain. Neurosci. Lett. 239:109–112.
Sluck, J. M., Lin, R. C. S., Katolik, L. I., Jeng, A. Y., and Lehmann, J. C. (1999). Endothelin converting enzime-1, endothelin-1 and endothelin-3 like immunoreactivity in the rat brain. Neuroscience 91:1483–1497.
Snyder, S. H., and Bredt, D. (1991). Nitric oxide as a neuronal messenger. Trends Pharmacol. Sci. 12:125–128.
Stanarius, A., Topel, I., Schulz, S., Noack, H., and Wolf, G. (1997). Immunocytochemistry of endothelial nitric oxide synthase in the rat brain: A light and electron microscopical study using the tyramide de signal amplification technique. Acta Histochem. 99:411–429.
Steiner, A., Parker, C., and Kipnis, D.M. (1972). Radio-immunoassay for cyclic nucleotides. J. Biol. Chem. 247:1106–1113.
Szabó, C. (1996) Physiological and pathophysiological roles of nitric oxide in the central nervous system. Brain Res. Bull. 41:131–141.
Tadepalli, A. S., and Hashim, M. A. (1995). Mechanisms of central endothelin-induced hypotension. Naunyn Schmiedebergs Arch. Pharmacol. 352:108–112.
Takai, M., Umemura, I., Yamasaki, K., Waranabe, T., Fujitani,Y., Oda, K., Urade,Y., Unui, T., Yamamura, T., and Okada, T. (1992). A potent and specific agonist, Suc-(Glu9, Ala11,15)-endothelin-1(18-21), IRL-1620, for ETB receptor. Biochem. Biophys. Res. Commun. 184:953–959.
Wall, K. M., and Ferguson, A. V. (1992). Brain Res. 586:111–116.
Yamada, K., Emson, P., and Hokfelt, T. (1996) Immunohistochemical mapping of nitric oxide synthase in the rat hypothalamus and colocalization with neuropeptides. J. Chem. Neuroanat. 10:295–316.
Yamada, H., and Kurokawa, K. (1998). Histochemical studies on endothelin and endothelin-A receptor in the hypothalamus. J. Cardiovasc. Pharmacol. 311(Suppl. 1):215–218.
Yamamoto, T., Suzuki, H., and Uemura, H. (1997). Endothelin B receptor-like immunoreactivity is associated with LHRH-immunoreactive fibers in the rat hypothalamus. Neurosci. Lett. 223:117–120.
Yamamoto, T., and Uemura, H. (1998). Distribution of endothelin B receptor-like immunoreactivity in the rat brain, kidney, and pancreas. J. Cardiovasc. Pharmacol. 311(Suppl. 1):S207–S211.
Yanagisawa, M., Kurihara, H., Kimura, S., Tomobe, Y., Kobayashi, M., Mitsui, Y., Goto, K., and Masaki, T. (1988). A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature 332:411–415.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Mathison, Y., Israel, A. Role of Endothelin Type B Receptor in NO/cGMP Signaling Pathway in Rat Median Eminence. Cell Mol Neurobiol 22, 783–795 (2002). https://doi.org/10.1023/A:1021817326632
Issue Date:
DOI: https://doi.org/10.1023/A:1021817326632