Abstract
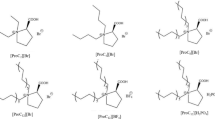
Semi-purified lipases from Candida rugosa, Pseudomonas cepacia and Alcaligenes sp. were chemically modified with a wide range of hydrophobic groups such as benzyloxycarbonyl, p-nitrobenzyloxycarbonyl, p-methoxybenzyloxycarbonyl, t-butoxycarbonyl, lauroyl and acetyl moieties. The Candida rugosa lipase MY modified with the benzyloxycarbonyl group (modification ratio = 84%) brought about a 15-fold increase in enantioselectivity (E value) towards the hydrolysis of racemic butyl 2-(4-ethylphenoxy)propionate in an aqueous buffer solution, although the enzymatic activity was decreased. The origin of the enantioselectivity enhancement by chemical modification of the lipase is attributed to a significant deceleration in the initial reaction rate for the incorrectly binding enantiomer.
Similar content being viewed by others
References
Bianchi D, Battistel E, Bosetti A, Cesti P, Fekete Z (1993) Effects of chemical modification on stereoselectivity of Pseudomonas cepacis lipase. Tetrahedron: Asymmetry 4: 777–782.
Carrea G, Riva S (2000) Properties and synthetic applications of enzymes in organic solvents. Angew. Chem. Int. Ed. Engl. 39: 2226–2254.
Chen C-S, Fujimoto Y, Girdaukas G, Sih CJ (1982) Quantitative analyses of biochemical kinetic resolutions of enantiomers. J. Am. Chem. Soc. 104: 7294–7299.
Fishman A, Basheer S, Shatzmiller S, Cogan U (1998) Fatty-acidmodified enzymes as enantioselective catalysts in microaqueous organic media. Biotechnol. Lett. 20: 535–538.
Gu Q-M, Sih CJ (1992) Improving the enantioselectivity of the Candida Cylindracea lipase VIA Chemical modification. Biocatalysis 6: 115–126.
Habeeb AFSA (1996) Determination of free amino groups in proteins by trinitrobenzenesulfonic acid. Anal. Biochem. 14: 328–336.
Okahata Y, Fujimoto Y, Ijiro K (1995) A lipid-coated lipase as an enantioselective ester synthesis catalyst in homogeneous organic solvent. J. Org. Chem. 60: 2244–2250.
Okamoto T, Ueji S (1999) Drastic enhancement of the enantioselectivity of lipase-catalysed esterification in organic solvents by the addition of metal ions. Chem. Commun. 939–940.
Okamoto T, Ueji S (2000) A new method for improving the enantioselectivity of lipase-catalyzed hydrolysis in organic solvent containing a small amount of water in the presence of metal ions. Biotechnol. Lett. 22: 1169–1171.
Overbeeke PLA, Koops BC, Verheiji HM, Slotboom AJ, Egmond MR, Jongejan JA, Heijnen JJ (2000) Activity and enantioselectivity of modified lipases in organic solvents. Biocatal. Biotranform. 18: 59–77.
Reetz MT (2002) Lipase as practical biocatalysts. Curr. Opin. Chem. Biol. 6: 145–150.
Schmid RD, Verger R (1998) Lipase: interfacial enzymes with attractive applications. Angew. Chem. Intern. Ed. Engl. 37: 1608–1633.
Sugai T (1999) Application of enzyme-and microorganismcatalyzed reactions to organic synthesis. Curr. Org. Chem. 3: 373–406.
Takahashi Y, Tanaka K, Murakami M, Kawanari M, Kawasaki Y, Tatsumi K, Okai H (1995) Characteristics of lipase modified with water-soluble acylating reagents and its esterification ability. Biosci. Biotech. Biochem. 59: 809–812.
Ueji S, Tanaka H, Ueda A, Watanabe K, Kaihatsu K, Ebara Y (2001a) Effects of chemical modification of lipase on its enantioselectivity in organic solvents. Chem. Lett. 1066–1067.
Ueji S, Nishimura M, Kudo R, Matsumi R, Watanabe K, Ebara Y (2001b) A dramatic improvement of enantioselectivity of lipase in organic solvents by addition of aqueous SDS: a close correlation between enantioselectivity and conformational flexibility of lipase. Chem. Lett. 912–913.
Watanabe K, Ueji S (2000) Dimethyl sulfoxide-induced high enantioselectivity of subtilisin Carlsberg for hydrolysis of ethyl 2-(4-substituted phenoxy)propionates. Biotechnol. Lett. 22: 599–603.
Watanabe K, Ueji S (2001) Dimethyl sulfoxide as a co-solvent dramatically enhances the enantioselectivity in lipase-catalysed resolutions of 2-phenoxypropionic derivatives. J. Chem. Soc. Perkin Trans. 1: 1386–1390.
Witiak DT, Ho TC-L, Hackney RE, Connor WE (1968) Hypocholesterolemic agents. Compounds related to ethyl ?-(4-chlorophenoxy)-?-methyl-propionate. J. Med. Chem. 11: 1086–1089.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ueji, Si., Ueda, A., Tanaka, H. et al. Chemical modification of lipases with various hydrophobic groups improves their enantioselectivity in hydrolytic reactions. Biotechnology Letters 25, 83–87 (2003). https://doi.org/10.1023/A:1021761508338
Issue Date:
DOI: https://doi.org/10.1023/A:1021761508338