Abstract
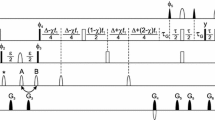
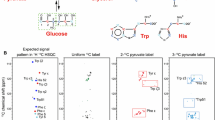
Analysis of 2D [13C,1H]-HSQC spectra of biosynthetic fractionally 13C labeled proteins is a reliable, straightforward means to obtain stereospecific assignments of Val and Leu methyl sites in proteins. Herein we show that the same fractionally labeled protein sample facilitates observation and identification of Phe and Tyr aromatic signals. This is the case, in part, because the fractional 13C labeling yields aromatic rings in which some of the 13C-13C J-couplings, present in uniformly labeled samples, are absent. Also, the number of homonuclear J-coupling partners differs for the δ-, ε- and ζ-carbons. This enabled us to vary their signal intensities in distinctly different ways by appropriately setting the 13C constant-time period in 2D [13C,1H]-HSQC spectra. We illustrate the application of this approach to an 18 kDa protein, c-VIAF, a modulator of apoptosis. In addition, we show that cancellation of the aromatic 13C CSA and 13C-1H dipolar interactions can be fruitfully utilized in the case of the fractionally labeled sample to obtain high resolution 13C constant-time spectra with good sensitivity.
Similar content being viewed by others
References
Delaglio, F., Grzesiek, S., Vuister, G.W., Zhu, G., Pfeifer, J. and Bax, A. (1995) J. Biomol. NMR,6, 277–293.
Goldman, M. (1984) J. Magn. Reson., 60, 437–452.
Neri, D., Szyperski, T., Otting, G., Senn, H. and Wüthrich, K. (1989) Biochemistry, 28, 7510–7516.
Pervushin, K., Braun, D., Fernandez, C. and Wüthrich, K. (2000) J. Biomol. NMR, 17, 195–202.
Pervushin, K., Riek, R., Wider, G. and Wüthrich, K. (1998)J. Am. Chem. Soc., 120, 6394–6400.
Senn, H., Werner, B., Messerle, B.A., Weber, C., Traber, R. and Wüthrich, K. (1989) FEBS Lett., 249, 113–118.
Szyperski, T. (1995) Eur. J. Biochem., 232, 433–448.
Szyperski, T., Neri, D., Leiting, B., Otting, G. and Wüthrich, K. (1992) J. Biomol. NMR., 2, 323-334.
Vuister, G.W. and Bax, A. (1992) J. Magn. Reson., 98, 428–435.
Vuister, G.W., Kim, S.J., Wu, C. and Bax, A.(1994) J. Am. Chem. Soc., 116, 9206–9210.
Wang, H., Janowick, D.A., Schkeryantz, J.M., Liu, X.H. and Fesik, S.W. (1999) J. Am. Chem. Soc., 121, 1611–1612.
Wishart, D.S., Bigam, C.G., Holm, A., Hodges, R.S. and Sykes, B.D. (1995) J. Biomol. NMR, 5, 67–81.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jacob, J., Louis, J.M., Nesheiwat, I. et al. Biosynthetically directed fractional 13C labeling facilitates identification of Phe and Tyr aromatic signals in proteins. J Biomol NMR 24, 231–235 (2002). https://doi.org/10.1023/A:1021662423490
Issue Date:
DOI: https://doi.org/10.1023/A:1021662423490