Abstract
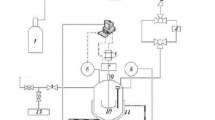
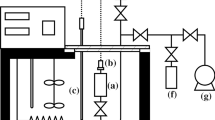
The dissociation of gas and model hydrates was studied using a classical thermodynamic method and a calorimetric method, in various aqueous media including pure water, high concentration calcium chloride solutions and water-in-oil emulsions. Methane hydrate dissociation temperatures vs. pressure curves were determined using pressure vs. temperature measurements in a constant volume cell (PVT), and high pressure differential scanning calorimetry (DSC), at 5 to 10 MPa gas pressure and at temperatures ranging from -10 to +12°C. PVT and DSC results are in good agreement, and concordant with data available in literature. From a thermodynamic point of view, there are no measurable differences between bulk solutions and emulsions. From a kinetic point of view, due to the considerable surface of interface between the two phases, emulsions allow the formation of much greater amounts of hydrate than solutions, without any agitation. Model hydrate of trichlorofluoromethane was studied in 9 to 27 mass% calcium chloride solutions in emulsion in oil, using DSC under atmospheric pressure, at temperatures ranging from -20 to +5°C. A diagram of dissociation temperature vs. salt concentration is proposed.
Similar content being viewed by others
References
E. Jr. D. Sloan, Clathrate Hydrates of Natural Gases, Marcel Dekker, New York 1990.
H. F. Fergusson, D. J. Frurip, A. J. Pastor, L. M. Peerey and L. F. Whiting, Thermochim. Acta, 363 (2000) 1.
Y. P. Handa, J. Chem. Thermodynamics, 18 (1986(a)) 915.
Y. P. Handa, Proc. AIChE Annual Meeting, Miami Beach, USA, 1986.
Y. P. Handa, Ind. Eng. Chem. Res., 27 (1988) 872.
C. A. Koh, R. E. Westacott, K. Hirachand, M. Zugic, W. Zhang and J. L. Savidge, Proc. Intern. Gas Res. Conf., San Diego, USA, 1 (1998) 194.
B. Fouconnier, V. Legrand, L. Komunjer, D. Clausse, L. Bergflodt and J. Sjöblom, Prog. Colloid Polym. Sci., 112 (1999) 105.
T. Jakobsen, J. Sjöblom and P. Ruoff, Colloids and Surfaces, 112 (1996) 73.
C. Weast, Handbook of Chemistry and Physics 67th Ed., CRC Press, Boca Raton, Florida, 1986.
C. Dalmazzone and D. Clausse, In ‘Encyclopaedic Handbook of Emulsion Technology’ Scientific Ed. J. Sjöblom. Chapter 14, ‘Microcalorimetry’ Marcel Dekker, New York 2001.
T. Hanai, K. Asami and N. Koizumi, Bull. Inst. Chem., 57 (1979) 197.
T. A. Wittstruck, W. S. Brey, A. M. Buswell and W. H. Rodebush, J. Chem. Eng. Data, 6 (1961) 343.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Dalmazzone, D., Kharrat, M., Lachet, V. et al. DSC and PVT measurements. Journal of Thermal Analysis and Calorimetry 70, 493–505 (2002). https://doi.org/10.1023/A:1021632709287
Issue Date:
DOI: https://doi.org/10.1023/A:1021632709287