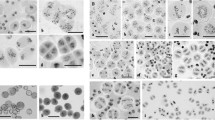
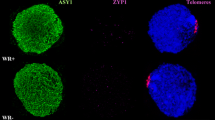
Abstract
Fluorescence in-situ hybridization (FISH) of total genomic and repetitive DNA on microsporocytes of ditelocentric addition lines of rye 5RL in hexaploid wheat was performed to study the behaviour of the rye homologous chromosome arms in relation to centromere and telomere dynamics at premeiotic interphase and meiotic prophase I. By comparing isogenic lines with and without the Ph1 locus, we established the effect of the Ph1 gene on appearance and behaviour of the rye chromosomes. Ph1 and ph1b lines demonstrated similar premeiotic chromosome arrangement with the two rye homologues occupying separated domains despite the occurrence of centromere association. Our study confirmed that bouquet arrangement of telomeres follows the Rabl configuration. In cells displaying bouquet clustering of telomeres, centromeres of the 5RL telosomes are still at the opposite pole, suggesting anchoring of centromeres at the cytoskeleton. Once the telomeres complete clustering, the rye centromeres migrate to the telomere pole, and the rye chromosomes begin to loosen their structure. While the rye homologues in the wild-type keep separate territories in the nucleus, they become intermingled in the ph1b mutant, possibly because of their lower condensation. In a subsequent stage, the 5RL homologues appear intimately associated mainly at the distal region. Our study suggests that the lower rate of chromosome synapsis in the ph1b mutant results from abnormal chromatin decondensation and organization.
Similar content being viewed by others
References
Abbo S, Dundorf RP, Foote T, Reader MS, Flavell RB, Moore G (1995) Organisation of retroelement and stem-loop repeat families in the genomes and nuclei of cereals. Chromosome Res 3: 5-15.
Armstrong SJ, Franklin FCH, Jones GH (2001) Nucleolus-associated telomere clustering and pairing precede meioticc hromosome synapsis in Arabidopsis thaliana. J Cell Sci 114: 4207-4217.
Aragón-Alcaide L, Miller T, Schwarzacher T, Reader S, Moore G (1996) A cereal centromeric sequence. Chromosoma 105: 261-268.
Aragón-Alcaide L, Reader S, Beven A, Shaw P, Miller T, Moore G (1997a) Association of homologous chromosomes during floral development. Current Biol 7: 905-908.
Aragón-Alcaide L, Reader S, Miller T, Moore G (1997b) Centromericbehavior in wheat with high and low homoeologous chromosomal pairing. Chromosoma 106: 327-333.
Bennett MD, Rao MK, Smith JB, Bayliss MW (1973) Cell development in the anther, the ovule and the young seed of Triticum aestivum L. var Chinese Spring. Phil Trans R Soc Lond B Biol Sci 266: 39-81.
Church K (1981) The architecture of and chromosome movements within the premeioticinterphase nucleus. In: Zimmerman AM, Forer A, eds. Mitosis/Cytokinesis. New York: Academic Press Inc, pp 83-102.
Croft JA, Bridger JM, Boyle S, Perry P, Teague P, Bickmore WA (1999) Differences in the localization and morphology of chromosomes in the human nucleus. J Cell Biol 145: 1119-1131.
Csink AK, Henikoff S (1998) Large-scale chromosomal movements during interphase progression in Drosophila. J Cell Biol 143: 13-22.
Darlington CD (1937) Recent Advances in Cytology, 2nd ed. Philadelphia: Blakiston.
Dover G, Riley R (1973) The effect of spindle inhibitors applied before meiosis on meioticc hromosome pairing. J Cell Sci 12: 143-161.
Dover GA, Riley R (1977) Inferences from genetical evidence on the course of meiotic chromosome pairing in plants. Phil Trans R Soc London Ser B 177: 313-326.
Driscoll CJ, Darvey NL (1970) Chromosome pairing effect of colchicine on an isochromosome. Science 169: 290-291.
Driscoll CJ, Darvey NL, Barber HN (1967) Effect of colchicine on meiosis of hexaploid wheat. Nature 216: 687-688.
Dvorak J, Lukaszewsky AJ (2000) Centromere association is an unlikely mechanism by which the wheat Ph1 locus regulates metaphase I chromosome pairing between homoeologous chromosomes. Chromosoma 109: 410-414.
Feldman M(1966) The effect of chromosomes 5B, 5D and 5A on chromosomal pairing in Triticum aestivum. Proc Natl Acad Sci USA 55: 1447-1453.
Feldman M (1993) Cytogenetic activity and mode of action of the pairing homoeologous (Ph1) gene of wheat. Crop Sci 33: 894-897.
Feldman M, Avivi L (1988) Geneticc ontrol of bivalent pairing in common wheat: the mode of Ph1 action In: Brandham PE, ed.
Kew Chromosome Conference III. London: HMSO, pp 269-279.
Fussell CP (1987) The Rabl orientation: a prelude to synapsis. In: Moens PB ed. Meiosis. Orlando: Academic Press, pp 275-299.
Gill KS, Gill BS, Endo TR, Mukai Y (1993) The physical mapping of Ph1, a chromosome pairing regulator gene in polyploid wheat. Genetics 134: 1231-1236.
Holm PB, Wang X (1988) The effect of chromosome 5B on synapsis and chiasma formation in wheat, Triticum aestivum cv. Chinese Spring. Carlsberg Res Commun 53: 191-208.
Loidl J (1990) The initiation of meiotic chromosome pairing: the cytological view. Genome 33: 759-778.
Lukaszewsky AJ (1997) The development and meiotic behavior of asymmetrical isochromosomes in wheat. Genetics 145: 1155-1160.
Martínez M, Naranjo T, Cuadrado C, Romero C (2001a) The synapticbehavior of Triticum turgidum with variable doses of the Ph1 locus. Theor Appl Genet 102: 751-758.
Martínez M, Cunado N, Carcelén N, Romero C (2001b) Ph1 and Ph2 loci play different roles in the synaptic behavior of hexaploid wheat Triticum aestivum. Theor Appl Genet 103: 398-405.
Martínez-Pérez E, Shaw P, Reader S et al. (1999) Homologous chromosome pairing in wheat. J Cell Sci 112: 1761-1769.
Martínez-Pérez, E, Shaw P, Moore G (2000) Polyploidy induces centromere association. J Cell Biol 148: 233-238.
Martínez-Pérez, E, Shaw P, Moore G (2001) The Ph1 locus is needed to ensure specific somaticand meiotic centromere association. Nature 411: 204-207.
McQuade HA, Bassett B (1977) Synaptonemal complexes in premeioticinterphase of pollen mother cells of Triticum aestivum. Chromosoma 63: 153-159.
Mikhailova EI, Naranjo T, Shepherd K et al. (1998) The effect of the wheat Ph1 locus on chromatin organization and meiotic pairing analysed by genome painting. Chromosoma 107: 339-350.
Qi L, Friebe B, Gill BS (2000) Meioticmetaphase I pairing behavior of a 5BL recombinant isochromosome in wheat. Chromosome Res 8: 671-676.
Richards EJ, Ausbel FM (1988) Isolation of a higher eukaryotic telomere sequence from Arabidopsis thaliana. Cell 53: 127-136.
Riley R, Chapman V (1958) Genetic control of the cytologically diploid behaviour of hexaploid wheat. Nature 182: 713-715.
Riley R, Chapman V (1964) The effect of the deficiency of the long arm of chromosome 5B on meiotic pairing in Triticum aestivum. Wheat Inf Serv 17: 12-15.
Roberts MA, Reader SM, Dalgliesh C et al. (1999) Induction and characterization of Ph1 wheat mutants. Genetics 153: 1909-1918.
Santos JL, Cuadrado MC, Díez M et al. (1995) Further insights on chromosomal pairing of autopolyploids: a triploid and tetraploids of rye. Chromosoma 104: 298-307.
Scherthan, H (1997) Chromosome behaviour in earliest meiotic prophase. Chromosomes Today: 12: 217-248.
Schwarzacher T (1997) Three stages of meiotic homologous chromosome pairing in wheat: cognition, alignment and synapsis. Sex Plant Reprod 10: 324-331.
Sears ER (1977) An induced mutant with homoeologous pairing in common wheat. Can J Genet Cytol 19: 585-593.
Sears ER, Okamoto M (1958) Intergenomic chromosome relationships in hexaploid wheat. Proc Tenth Int Cong Genet 2: 258-259.
Sybenga J (1992) Cytogenetics in Plant Breeding. Heildelberg, Berlin, New York: Springer.
Upadhya MD, Swaminathan MS (1967) Mechanisms regulating chromosome pairing in Triticum. Biol Zbl 86: 239-255.
Vega JM, Feldman M (1998) Effect of the pairing gene Ph1 and premeiotic colchicine treatment on intra-and interchromosome pairing of isochromosomes in common wheat. Genetics 150: 1199-1208.
Zhong X, Fransz P, Wennekes van Eden J et al. (1996) High-resolution mapping on pachytene chromosomes and extended DNA fibers by fluorescent in situ hybridisation. Plant Mol Biol Rep 14: 232-242.
Zickler D, Kleckner N (1998) The leptotene-zygotene transition of meiosis. Annu Rev Genet 32: 619-697.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Maestra, B., de Jong, J.H., Shepherd, K. et al. Chromosome arrangement and behaviour of two rye homologous telosomes at the onset of meiosis in disomic wheat-5RL addition lines with and without the Ph1 locus. Chromosome Res 10, 655–667 (2002). https://doi.org/10.1023/A:1021564327226
Issue Date:
DOI: https://doi.org/10.1023/A:1021564327226