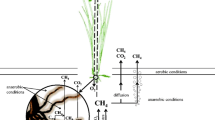
Abstract
Acidic Sphagnum peat bogs cover a considerable part of the territory of Russia and are an important natural source of biogenic methane, which is formed in their anaerobic layers. A considerable portion of this methane is consumed in the aerobic part of the bog profile by acidophilic methanotrophic bacteria, which comprise the methane filter of Sphagnum peat bogs and decrease CH4 emission to the atmosphere. For a long time, these bacteria escaped isolation, which became possible only after the elucidation of the optimal conditions of their functioning in situ: pH 4.5–5.5; temperature, from 15 to 20°C; and low salt concentration in the solution. Imitation of these conditions and rejection of earlier used media with a high content of biogenic elements allowed methanotrophic bacteria of two new genera and species—Methylocella palustris and Methylocapsa acidiphila—to be isolated from the peat of Sphagnum peat bogs of European northern Russia and western Siberia. These bacteria are well adapted to the conditions in cold, acidic, oligotrophic Sphagnum peat bogs. They grow in a pH range of 4.2–7.5 with an optimum at 5.0–5.5, prefer moderate temperatures (15–25°C) and media with a low content of mineral salts (200–500 mg/l), and are capable of active dinitrogen fixation. Design of fluorescently labeled 16S rRNA–targeted oligonucleotide probes for the detection of Methylocella palustris and Methylocapsa acidiphila and their application to the analysis of sphagnum peat samples showed that these bacteria represent dominant populations of methanotrophs with a density of 105–106 cells/g peat. In addition to Methylocella and Methylocapsa populations, one more abundant population of methanotrophs was revealed (106 cells/g peat), which were phylogenetically close to the genus Methylocystis.
Similar content being viewed by others
REFERENCES
Cicerone, R. and Oremland, R., Biogeochemical Aspects of Atmospheric Methane, Global Biogeochem. Cycles, 1988, vol. 2(4), pp. 299–327.
Matthews, E. and Fung, I., Methane Emissions from Natural Wetlands: Global Distribution, Area, and Environmental Characteristics of Sources, Global Biogeochem. Cycles, 1987, vol. 1, pp. 61–86.
Aselmann, I. and Crutzen, P.J., Global Distribution of Natural Freshwater Wetlands and Rice Paddies, Their Net Primary Productivity, Seasonality and Possible Methane Emissions, J. Atmos. Chemistry, 1989, vol. 8, pp. 307–358.
Hein, R., Crutzen, P.J., and Heinmann, M., An Inverse Modeling Approach to Investigate the Global Atmospheric Methane Cycle, Global Biogeochem. Cycles, 1997, vol. 11(1), pp. 43–76.
Panikov, N.S., Fluxes of CO2 and CH4 in High Latitude Wetlands: Measuring, Modeling and Predicting Response to Climate Change, Polar Research, 1999, vol. 18(2), pp.-237–244.
Zavarzin, G.A. and Vasil'eva, L.V., Methane Cycle on the Territory of Russia, Krugovorot ugleroda na territorii Rossii (Carbon Cycle on the Territory of Russia), Moscow: Izd-vo Mosk. Pravitel'stva, 1999, pp. 202–230.
Land Resources of Russia. CD-ROM Database Created by International Institute for Applied Systems Analysis, Russ. Acad. Sci., 2002.
Panikov, N.S., Titlyanova, A.A., Paleeva, M.V., Semenov, A.M., Mironycheva-Tokareva, N.P., Makarov, V.I., Dubinin, E.V., and Efremov, S.P., Methane Emission from Bogs of West Siberia), Dokl. Akad. Nauk, 1993, vol. 330(3), pp. 388–390.
Panikov, N.S., Sizova, M.V., Zelenev, V.V., Makhov, G.A., Naumov, A.V., and Gadzhiev, I.M., Emission of CH4 and CO2 from Bogs of the South of West Siberia: Spatial and Temporal Variations in the Fluxes, Zh. Ekol. Chem., 1995, vol. 4, pp. 13–24.
Panikov, N.S., Glagolev, M.V., Kravchenko, I.K., Mastepanov, M.A., Kosykh, N.P., Mironycheva-Tokareva, N.P., Naumov, A.V., Inoue, G., and Maksyutov, Sh., Methane Emission from Ombrotrophic Bogs of West Siberia as Dependent on the Type of Vegetation Cover, Zh. Ekol. Chem., 1997, vol. 6, no. 1, pp. 59–67.
Zavarzin, G.A., Psychrophilic Soengen Cycle, Ekol. Khim., 1995, vol. 4(1), p. 3.
Henckel, T., Friedrich, M., and Conrad, R., Molecular Analyses of the Methane-Oxidizing Microbial Community in Rice Field Soil by Targeting the Genes of the 16S rRNA, Particulate Methane Monooxygenase, and Methanol Dehydrogenase, Appl. Environ. Microbiol., 1999, vol. 66, no. 5, pp. 1980–1990.
Frenzel, P., Plant-Associated Methane Oxidation in Rice Fields and Wetlands, Adv. Microb. Ecol., 2000, pp. 85–114.
Henckel, T., Jackel, U., and Conrad, R., Vertical Distribution of the Methanotrophic Community after Drainage of Rice Field Soil, FEMS Microbiol. Ecol., 2001, vol. 34, pp. 279–291.
Eller, G. and Frenzel, P., Changes in Activity and Community Structure of Methane-Oxidizing Bacteria over the Growth Period of Rice, Appl. Environ. Microbiol., 2001, vol. 67, pp. 2395–2403.
Namsaraev, B.B., Microbial Interactions during Methane Oxidation, Cand. Sci. (Biol.) Dissertation, Moscow, 1973.
Yavitt, J.B., Lang, G.E., and Downey, D.M., Potential Methane Production and Methane Oxidation Rates in Peatland Ecosystems of the Appalachian Mountains, United States, Global Biogeochem. Cycles, 1988, vol. 2, no. 3, pp. 253–268.
Yavitt, J.B., Downey, G.E., Lancaster, E., and Lang, G.E., Methane Consumption in Decomposing Sphagnum-Derived Peat, Soil Biol. Biochem., 1990, vol. 22, pp. 441–447.
Panikov, N.S., Semenov, A.M., Tarasov, A.A., Belyaev, A.S., Kravchenko, I.K., Smagina, M.V., Palejeva, M.V., Zelenev, V.V., and Skupchenko, K.V., Methane Production and Uptake in Soils of the European Part of the USSR, J. Ecol. Chem., 1993, no. 1, pp. 7–18.
Sundh, I., Nilsson, M., Granberg, G., and Svensson, B.H., Depth Distribution of Microbial Production and Oxidation of Methane in Northern Boreal Peatlands, Microb. Ecol., 1994, vol. 27, pp. 253–265.
Sundh, I., Mikkela, C., Nilsson, M., and Svensson, B.H., Potential Aerobic Methane Oxidation in a Sphagnum-Dominated Peatland-Controlling Factors and Relation to Methane Emission, Soil Biol. Biochem., 1995, vol. 27, pp. 829–837.
Nedwell, D.B. and Watson, A., CH4 Production, Oxidation and Emission in a UK Ombrotrophic Peat Bog: Influence of 2-4 from Acid Rain, Soil Biol. Biochem., 1995, vol. 27, pp. 893–903.
Krumholz, L.R., Hollenback, J.L., Roskes, S.J., and Ringelberg, D.B., Methanogenesis and Methanotrophy within a Sphagnum Peatland, FEMS Microbiol. Ecol., 1995, vol. 18, pp. 215–224.
McDonald, I.R., Hall, G.H., Pickup, R.W., and Murrell, J.C., Methane Oxidation Potential and Preliminary Analysis of Methanotrophs in Blanket Bog Peat Using Molecular Ecology Techniques, FEMS Microbiol. Ecol., 1996, vol. 21, pp. 197–211.
Prior, S.D. and Dalton, H., Acetylene as a Suicide Substrate and Active Site Probe for Methane Monooxygenase from Methylococcus capsulatus (Bath), FEMS Microbiol. Lett., 1985, vol. 29, pp. 105–109.
Bedard, C. and Knowles, R., Physiology, Biochemistry and Specific Inhibitors of CH4, NH +4 And CO2 Oxidation by Methylotrophs and Nitrifiers, Microbiol. Rev., 1989, vol. 53, no. 1, pp. 68–84.
Dedysh, S.N. and Panikov, N.S., Effect of Methane Concentration on the Rate of Its Oxidation by Bacteria in Sphagnum Peat, Mikrobiologiya, 1997, vol. 66, pp. 563–568.
Vasil'eva, L.V., Berestovskaya, Yu.Y., and Zavarzin, G.A., Psychrophilic Acidophilic Methanotrophs from Sphagnetta of Permafrost Zone, Dokl. Akad. Nauk, 1999, vol. 368, no. 1, pp. 125–128.
Dunfield, P., Knowles, R., Dumont, R., and Moore, T.R., Methane Production and Consumption in Temperate and Subarctic Peat Soils: Response to Temperature and pH, Soil Biol. Biochem., 1993, vol. 25, no. 3, pp. 321–326.
Dedysh, S.N. and Panikov, N.S., Effect of pH, Temperature, and Concentration of Salts on Methane Oxidation Kinetics in Sphagnum Peat, Mikrobiologiya, 1997, vol. 66, pp. 569–573.
Hanson, R.S. and Hanson, T.E., Methanotrophic Bacteria, Microbiol. Rev., 1996, vol. 60, no. 2, pp. 439–471.
Gal'chenko, V.F., Metanotrofnye bakterii (Methanotrophic Bacteria), Moscow: GEOS, 2001.
Whittenbury, R., Phillips, K.C., and Wilkinson, T.F., Enrichment, Isolation and Some Properties of Methane-Utilizing Bacteria, J. Gen. Microbiol., 1970, vol. 61, pp.-205–218.
Bowman, J.P., Sly, L.I., and Stackebrandt, E., The Phylogenetic Position of the Family Methylococcaceae, Int. J. Syst. Bacteriol., 1995, vol. 45, pp. 182–185.
Bowman, J.P., McCammon, S.A., and Skerratt, J.H., Methylosphaera hansonii gen. nov., sp. nov., a Psychrophilic, Group I Methanotroph from Antarctic Marine-Salinity, Meromictic Lakes, Microbiology, 1997, vol. 143, pp. 1451–1459.
Bodrossy, L., Holmes, E.M., Holmes, A.J., Kovacs, K.L., and Murrell, J.C., Analysis of 16S rRNA and Methane Monooxygenase Gene Sequences Reveals a Novel Group of Thermotolerant and Thermophilic Methanotrophs, Methylocaldum gen. nov., Arch. Microbiol., 1997, vol. 168, pp. 493–503.
Wise, M.G., McArthur, J.V., and Shimkets, L.J., Methylosarcina fibrata gen. nov., sp. nov. and Methylosarcina quisquiliarum sp. nov., Novel Type I Methanotrophs, Int. J. Syst. Evol. Microbiol., 2001, vol. 51, pp. 611–621.
Dedysh, S.N., Liesack, W., Khmelenina, V.N., Suzina, N.E., Trotsenko, Y.A., Semrau, J.D., Bares, A.M., Panikov, N.S., and Tiedje, J.M., Methylocella palustris gen. nov., sp. nov., a New Methane-Oxidizing Acidophilic Bacterium from Peat Bogs Representing a Novel Sub-Type of Serine Pathway Methanotrophs, Int. J. Syst. Evol. Microbiol., 2000, vol. 50, pp. 955–969.
Dedysh, S.N., Khmelenina, V.N., Suzina, N.E., Trotsenko, Y.A., Semrau, J.D., Liesack, W., and Tiedje, J.M., Methylocapsa acidiphila gen. nov., sp. nov., a Novel Methane-Oxidizing and Dinitrogen-Fixing Acidophilic Bacterium from Sphagnum Bog, Int. J. Syst. Evol. Microbiol., 2002, vol. 52, pp. 251–261.
Omel'chenko, M.V., Savel'eva, N.D., Vasil'eva, L.V., and Zavarzin, G.A., Psychrophilic Methanotrophic Community from Tundra Soil, Mikrobiologiya, 1992, vol. 61, no. 6, pp. 1072–1077.
Omel'chenko, M.V., Vasil'eva, L.V., Zavarzin, G.A., Savel'eva, N.D., Lysenko, A.M., Mityushina, L.L., Khmelenina, V.N., and Trotsenko, Yu.A., A Novel Psychrophilic Methanotroph of the Genus Methylobacter, Mikrobiologiya, 1996, vol. 65, no. 3, pp. 384–389.
Butorova, I.A., Microbial Community on Natural Gas ans Possibilities of Its Regulation, Cand. Sci. (Biol.) Dissertation, Leningrad, 1991.
Malashenko, Yu.P., Romanovskaya, V.A., and Trotsenko, Yu.A., Metanokislyayushchie mikroorganizmy (Methane-Oxidizing Microorganisms), Moscow: Nauka, 1978.
Gal'chenko, V.F., Andreev, L.V., and Trotsenko, Yu.A., Taksonomiya i identifikatsiya obligatnykh metanotrofnykh bakterii (Taxonomy and Identification of Obligately Methanotrophic Bacteria), Pushchino: Otd. Nauchn. Tekh. Inform. Nauchn. Tsentr Biol. Inf. Akad. Nauk, SSSR, 1986.
Topp, E. and Hanson, R.S., Metabolism of Radiatively Important Trace Gases by Methane-Oxidizing Bacteria, Microbial Production and Consumption of Greenhouse Gases: Methane, Nitrogen Oxides and Halomethanes, Rogers, J.E. and Whitman, W.B., Eds., Washington, D.C.: Am. Soc. Microbiol., 1991, pp. 71–90.
King, G.M., Ecological Aspects of Methane Oxidation, a Key Determinant of Global Methane Dynamics, Adv. Microb. Ecol., 1992, vol. 12, pp. 431–461.
Heyer, J. and Suckow, R., Ecological Studies of Methane Oxidation in an Acid Bog Lake, Limnologica, 1985, vol. 16, pp. 247–266.
Murrell, J.C., McDonald, I.R., and Bourne, D.J., Molecular Methods for the Study of Methanotrophs Ecology, FEMS Microbiol. Ecol., 1998, vol. 27, pp. 103–114.
Murrell, J.C. and Radajewski, S., Cultivation-Independent Techniques for Studying Methanotroph Ecology, Res. Microbiol., 2000, vol. 151, pp. 807–814.
McDonald, I.R. and Murrell, J.C., The Methanol Dehydrogenase Structural Gene mxaF and Its Use as a Functional Gene Probe for Methanotrophs and Methylotrophs, Appl. Environ. Microbiol., 1997, vol. 63, pp. 3218–3224.
McDonald, I.R. and Murrell, J.C., The Particulate Methane Monooxygenase Gene pmoA and Its Use as a Functional Gene Probe for Methanotrophs, FEMS Microbiol. Lett., 1997, vol. 156, pp. 205–210.
Andreev, L.V. and Gal'chenko, V.F., Fatty-Acid Composition and Identification of Methanotrophic Bacteria, Dokl. Akad. Nauk SSSR, 1978, vol. 239, pp. 1465–1468.
Guckert, J.B., Ringelberg, D.B., White, D.C., Hanson, R.S., and Bratina, B.J., Membrane Fatty Acids as Phenotypic Markers in the Polyphasic Taxonomy of Methylotrophs within the Proteobacteria, J. Gen. Microbiol., 1991, vol. 137, pp. 2631–2641.
Bowman, J.P., Sly, L.I., Nichols, P.D., and Hayward, A.C., Revised Taxonomy of the Methanotrophs: Description of the Methylobacter gen. nov., Emendation of Methylococcus, Validation of Methylosinus and Methylocystis Species, and a Proposal That the Family Methylococcaceae Includes Only the Group I Methanotrophs, Int. J. Syst. Bacteriol., 1993, vol. 43, pp. 735–753.
Bowman, J.P., Skerratt, J.H., Nichols, P.D., and Sly, L.I., Phospholipid Fatty Acid and Lipopolysaccharide Fatty Acid Signature Lipids in Methane-Utilizing Bacteria, FEMS Microbiol. Ecol., 1991, vol. 85, pp. 15–22.
Sundh, I., Borda, P., Nilsson, M., and Svensson Bo, H., Estimation of Cell Numbers of Methanotrophic Bacteria in Boreal Peatlands Based on Analysis of Specific Phospholipid Fatty Acids, FEMS Microbiol. Ecol., 1995, vol. 18, pp. 103–112.
Sundh, I., Nilsson, M., and Borga, P., Variation in Microbial Community Structure in Two Boreal Peatlands as Determined by Analysis of Phospholipid Fatty Acid Profiles, Appl. Environ. Microbiol., 1997, vol. 63, pp. 1476–1482.
Dedysh, S.N., Panikov, N.S., and Tiedje, J.M., Acidophilic Methanotrophic Communities from Sphagnum Peat Bogs, Appl. Environ. Microbiol., 1998, vol. 64, pp.-922–929.
McDonald, I.R., Kenna, E.M., and Murrell, J.C., Detection of Methanotrophic Bacteria in Environmental Samples with the PCR, Appl. Environ. Microbiol., 1995, vol. 61, pp. 116–121.
Holmes, A.J., Costello, A., Lidstrom, M.E., and Murrell, J.C., Evidence That Particulate Methane Monooxygenase and Ammonia Monooxygenase May Be Evolutionarily Related, FEMS Microbiol. Lett., 1995, vol. 132, pp. 203–208.
Shigematsu, T., Hanada, S., Eguchi, M., Kamagata, Y., Kanagawa, T., and Kurane, R., Soluble Methane Monooxygenase Gene Clusters from Trichloroethylene-Degrading Methylomonas sp. Strains and Detection of Methanotrophs during In Situ Bioremediation, Appl. Environ. Microbiol., 1999, vol. 65, pp. 5198–5206.
Dedysh, S.N., Liesack, W., Horz, H.-P., and Tiedje, J., A Novel Methane-Oxidizing Strain from Methanotrophic Community Capable of Growth at pH 4, Abstr. Kongress der VAAM Mikrobiologie 2000, 2000, Munchen, Germany, 12-16.III, p. 147.
Dedysh, S.N., Horz, H.-P., Dunfield, P.F., and Liesack, W., A Novel pmoA Lineage Represented by the Acidophilic Methanotrophic Bacterium Methylocapsa acidiphila B2, Arch. Microbiol., 2001, vol. 177, pp.-117–121.
Dedysh, S.N., Panikov, N.S., Liesack, W., Grokopf, R., Zhou, J., and Tiedje, J.M., Isolation of Acidophilic Methane-Oxidizing Bacteria from Northern Peat Wetlands, Science, 1998, vol. 282, pp. 281–284.
Conti, S.F. and Hirsch, P., Biology of Budding Bacteria: III. Fine Structure of Rhodomicrobium and Hyphomicrobium spp., J. Bacteriol., 1965, vol. 89, pp. 503–512.
Oelze, J. and Drews, G., Membranes of Photosynthetic Bacteria, Biochim. Biophys. Acta, 1971, vol. 265, pp.-209–239.
Holmes, A.J., Roslev, P., McDonald, I.R., Iversen, N., Henriksen, K., and Murrell, J.C., Characterization of Methanotrophic Bacterial Populations in Soils Showing Atmospheric Methane Uptake, Appl. Environ. Microbiol., 1999, vol. 65, pp. 3312–3318.
Henckel, T., Jackel, U., Schnell, S., and Conrad, R., Molecular Analyses of Novel Methanotrophic Communities in Forest Soil Oxidizing Atmospheric Methane, Appl. Environ. Microbiol., 2000, vol. 66, pp. 1801–1808.
Jensen, S., Holmes, A.J., Olsen, R.A., and Murrell, J.C., Detection of Methane Oxidizing Bacteria in Forest Soil by Monooxygenase PCR Amplification, Microb., Ecol., 2000, vol. 39, pp. 282–289.
Dalton, H., Methane Oxidation by Methanotrophs: Physiological and Mechanistic Implications, Methane and Methanol Utilizers, Murrell, J.C. and Dalton, H., Eds., New York: Plenum, 1992, pp. 85–114.
Semrau, J.D., Zolandz, D., Lidstrom, M.E., and Chan, S.I., The Role of Copper in the pMMO of Methylococcus capsulatus (Bath): a Structural Vs Catalytic Function, J. Inorg. Biochem., 1995, vol. 58, pp. 235–244.
Murrell, J.C., Gilbert, B., and McDonald, I.R., Molecular Biology and Regulation of Methane Monooxygenase, Arch. Microbiol., 2000, vol. 173, pp. 325–332.
Stanley, S.H., Prior, S.D., Leak, D.J., and Dalton, H., Copper Stress Underlies the Fundamental Change in Intracellular Location of Methane Mono-Oxygenase in Methane-Oxidizing Organisms: Studies in Batch and Continuous Cultures, Biotechnol. Lett., 1983, vol. 5, pp.-487–492.
Richardson, C.J., Biogeochemical Cycles: Regional, Wetlands and Shallow Continental Water Bodies, Patten, B.C., Ed., Hague: SPB Acad., 1990, pp. 259–279.
Radajewski, S., Ineson, P., Parekh, N.R., and Murrell, J.C., Stable-Isotope Probing as a Tool in Microbial Ecology, Nature (London), 2000, vol. 403, pp. 646–649.
Costello, A. and Lidstrom, M.E., Molecular Characterization of Functional and Phylogenetic Genes from Natural Populations of Methanotrophs in Lake Sediments, Appl. Environ. Microbiol., 1999, vol. 65, pp. 5066–5074.
Wise, M.G., McArthur, J.V., and Shimkets, L.J., Methanotroph Diversity in Landfill Soil: Isolation of Novel Type I and Novel Type II Methanotrophs Whose Presence Was Suggested by Culture-Independent 16S Ribosomal DNA Analysis, Appl. Environ. Microbiol., 1999, vol. 65, pp. 4887–4897.
Holmes, A.J., Owens, N.J.P., and Murrell, J.C., Detection of Novel Marine Methanotrophs Using Phylogenetic and Functional Gene Probes after Methane Enrichment, Microbiology (UK), 1995, vol. 141, pp. 1947–1955.
Auman, A.J., Stolyar, S., Costello, A.M., and Lidstrom, M.E., Molecular Characterization of Methanotrophic Isolates from Freshwater Lake Sediment, Appl. Environ. Microbiol., 2000, vol. 66, pp. 5259–5266.
Tsien, H.C., Bratina, B.J., Tsuji, K., and Hanson, R.S., Use of Oligodeoxynucleotide Signature Probes for Identification of Physiological Groups of Methylotrophic Bacteria, Appl. Environ. Microbiol., 1990, vol. 56, pp.-2858–2865.
Bourne, D.G., Holmes, A.J., Iversen, N., and Murrell, J.C., Fluorescent Oligonucleotide rDNA Probes for Specific Detection of Methane Oxidizing Bacteria FEMS Microbiol. Ecol., 2000, vol. 31, pp. 29–38.
Eller, G., Stubner, S., and Frenzel, P., Group Specific 16S rRNA Targeted Probes for the Detection of Type I and Type II Methanotrophs by Fluorescence In Situ Hybridisation, FEMS Microbiol. Lett., 2001, vol. 198, pp. 91–97.
Dedysh, S.N., Derakshani, M., and Liesack, W., Detection and Enumeration of Methanotrophs in Acidic Sphagnum Peat by 16S rRNA Fluorescence In Situ Hybridization, Including the Use of Newly Developed Oligonucleotide Probes for Methylocella palustris, Appl. Environ. Microbiol., 2001, vol. 67, pp. 4850–4857.
Dedysh, S.N., Dunfield, P.F., Derakshani, M., Stubner, S., Heyer, J., and Liesack, W., Differential Detection of Type II Methanotrophic Bacteria in Acidic Peatlands Using Newly Developed 16S rRNA-targeted Fluorescent Oligonucleotide probes, FEMS Microbiol. Ecol., 2002 (accepted).
Amann, R.I., Krumholz, L., and Stahl, D.A., Fluorescent-Oligonucleotide Probing of Whole Cells for Determinative, Phylogenetic, and Environmental Studies in Microbiology, J. Bacteriol., 1990, vol. 172, pp. 762–770.
Amann, R.I., Ludwig, W., and Schleifer, K.-H., Phylogenetic Identification and In Situ Detection of Individual Microbial Cells without Cultivation, Microbiol. Rev., 1995, vol. 59, pp. 143–169.
Moter A. and Gobel, U.B., Fluorescence In Situ Hybridization (FISH) for Direct Visualization of Microorganisms, J. Microbiol. Methods, 2000, vol. 41, pp. 85–112.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Dedysh, S.N. Methanotrophic Bacteria of Acidic Sphagnum Peat Bogs. Microbiology 71, 638–650 (2002). https://doi.org/10.1023/A:1021467520274
Issue Date:
DOI: https://doi.org/10.1023/A:1021467520274