Abstract
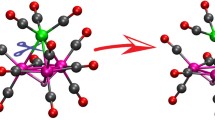
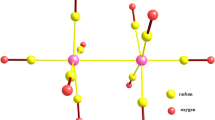
Quantum-chemical calculations of ferrocenylmethyl ([C5H5FeC5H4CH2]+) and ferrocenylenedimethyl ([C5H5FeC5H3(CH2)2]2+) cations with full geometry optimization were carried out using the Hartree—Fock (HF) approximation, density functional theory (DFT), and at the second-order Møller—Plesset (MP2) level of perturbation theory in the 6-311G* basis set. The methods with inclusion of electron correlation in explicit form indicate that the CH2groups deviate from the cyclopentadienyl ring planes toward the Fe atom due to formation of the Fe—CH2bonds. According to Hartree—Fock calculations, ligands in these ions are virtually planar. The metallonium character of the ions studied was demonstrated based on the results of analysis of the electron density distribution and frontier orbitals.
Similar content being viewed by others
References
M. I. Rybinskaya, A. Z. Kreindlin, and S. S. Fadeeva, J. Organomet. Chem., 1988, 358, 363.
M. I. Rybinskaya, A. Z. Kreindlin, Yu. T. Struchkov, and A. I. Yanovsky, J. Organomet. Chem., 1989, 359, 233.
A. Z. Kreindlin, F. M. Doldushin, A. I. Yanovsky, Z. E. Kerzina, P. V. Petrovskii, and M. I. Rybinskaya, J. Organomet. Chem., 2000, 616, 106.
A. Z. Kreindlin, E. I. Fedin, P. V. Petrovskii, M. I. Rybinskaya, R. M. Minyaev, and R. Hoffmann, Organometallics, 1991, 10, 1206.
M. I. Rybinskaya, A. Z. Kreindlin, P. V. Petrovskii, R. M. Minyaev, and R. Hoffmann, Organometallics, 1994, 13, 3903.
M. I. Rybinskaya, A. Z. Kreindlin, R. Hoffmann, and R. M. Minyaev, Izv. Akad. Nauk, Ser. Khim., 1994, 1701 [Russ. Chem. Bull., 1994, 43, 1605 (Engl. Transl.)].
A. A. Kamyshova, A. Z. Kreindlin, M. I. Rybinskaya, R. Hoffmann, and P. V. Petrovskii, Izv. Akad. Nauk, Ser. Khim., 1999, 587 [Russ. Chem. Bull., 1999, 48, 581 (Engl. Transl.)].
A. A. Kamyshova, A. Z. Kreindlin, M. I. Rybinskaya, and P. V. Petrovskii, Izv. Akad. Nauk, Ser. Khim., 2000, 370 [Russ. Chem. Bull., Int. Ed., 2000, 49, 372].
W. E. Watts, J. Organomet. Chem. Libr., 1979, 7, 399
A. A. Koridze, Usp. Khim., 1986, 55, 277 [Russ. Chem. Rev., 1986, 55(Engl. Transl.)].
H. P. Luthi, J. H. Ammeter, J. Almløf, and K. Faegri, J. Chem. Phys., 1982, 77, 2002.
C. Park and J. Almlöf, J. Chem. Phys., 1991, 95, 1829.
M. L. McKee, J. Am. Chem. Soc., 1993, 115, 2818.
S. Niu and M. B. Hall, Chem. Rev., 2000, 100, 353.
E. G. Gal´pern, N. P. Gambaryan, A. Z. Kreindlin, M. I. Rybinskaya, I. V. Stankevich, and A. L. Chistyakov, Metalloorg. Khim., 1992, 5, 401 [Organomet. Chem. USSR, 1992, 5(Engl. Transl.)].
M. I. Rybinskaya, Y. S. Nekrasov, Y. A. Borisov, A. I. Belokon´, A. Z. Kreindlin, A. A. Kamyshova, and N. V. Kruglova, J. Organomet. Chem., 2001, 631, 9.
A. D. Becke, J. Chem. Phys., 1993, 98, 5648.
C. Lee, W. Yang, and R. G. Parr, Phys. Rev., B, 1988, 150, 785.
Per E. M. Siegbahn and M. R. A. Blomberg, Chem. Rev., 2000, 100, 421.
M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, V. G. Zakrzewski, J. A. Montgomery, Jr., R. E. Stratmann, J. C. Burant, S. Dapprich, J. M. Millam, A. D. Daniels, K. N. Kudin, M. C. Strain, O. Farkas, J. Tomasi, V. Barone, M. Cossi, R. Cammi, B. Mennucci, C. Pomelli, C. Adamo, S. Clifford, J. Ochterski, G. A. Petersson, P. Y. Ayala, Q. Cui, K. Morokuma, D. K. Malick, A. D. Rabuck, K. Raghavachari, J. B. Foresman, J. Cioslowski, J. V. Ortiz, B. B. Stefanov, G. Liu, A. Liashenko, P. Piskorz, I. Komaromi, R. Gomperts, R. L. Martin, D. J. Fox, T. Keith, M. A. Al-Laham, C. Y. Peng, A. Nanayakkara, C. Gonzalez, M. Challacombe, P. M. W. Gill, B. Johnson, W. Chen, M. W. Wong, J. L. Andres, C. Gonzalez, M. Head-Gordon, E. S. Replogle, and J. A. Pople, GAUSSIAN-98, REVISION A.5, Gaussian, Inc., Pittsburgh (PA), 1998.
R. K. Bohn and A. Haaland, J. Organomet. Chem., 1966, 5, 470.
A. I. Yanovsky, Yu. T. Struchkov, A. Z. Kreindlin, and M. I. Rybinskaya, J. Organomet. Chem., 1989, 359, 125.
Molekulyarnye postoyannye neorganicheskikh soedinenii[Molecular Constants of Inorganic Compounds], Ed. K. S. Krasnov, Khimiya, Leningrad, 1979, 448 pp. (in Russian).
Yu. A. Borisov, M. I. Rybinskaya, Yu. S. Nekrasov, A. Z. Kreindlin, A. A. Kamyshova, and P. V. Petrovskii, J. Organomet. Chem., 2002, 645, 87.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Borisov, Y.A., Nekrasov, Y.S., Rybinskaya, M.I. et al. Nature of Fe—CH2 bonds in ferrocenylmethyl and ferrocenylenedimethyl cations. Russian Chemical Bulletin 51, 1651–1655 (2002). https://doi.org/10.1023/A:1021387018591
Issue Date:
DOI: https://doi.org/10.1023/A:1021387018591