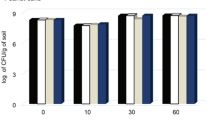
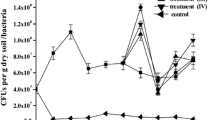
Abstract
Batch enrichment cultures for creosote catabolizing microorganisms was carried out using a creosote-contaminated soil as the inoculum. The flasks were separately spiked with phenol, o-cresol, m-cresol, p-cresol, naphthalene, anthracene, phenanthrene, pyrrole, fluorene, pyrene, fluoranthene, chrysene,benzo(a)pyrene and creosote (a complex mixture of about 400 compounds) in concentrations of 50, 100, 500, 1000, 5000, 10 000, 15 000, 20 000, 25 000 and 30 000 mg L-1. The flasks were incubated on a rotary shaker in the dark at 30 °C. Samples for analysis were taken from the flasks every three days for three weeks. Counts of microorganisms were observed to be highest in most cases in 5000 mg L-1 cultures. The pH values were observed to fluctuate between 5 and8 but this did not seem to affect the growth of the organisms except at 50 and 100 mg L-1 in the phenolics and some polycyclic aromatic hydrocarbons (PAHs) were the decreases correlated with decreases in cell counts. The isolates in the first week were mainly of one morphological type in most cultures. In subsequent weeks, the populations became mixed. The higher molecular mass PAHs at higher concentration continued to support only a few types of organisms. Isolates included bacteria, actinomycetes and fungi. The phenolics and naphthalene were more readily removed (up to 100%) from the cultures. Other hydrocarbons removal were between 45 and 83% with the higher molecular mass compounds being most recalcitrant. The decreases in creosote concentration was similar to those in the phenolic compounds and the lower molecular mass compounds up to a concentration of 10 000mg L-1. Decreases in creosote concentration started to decrease from a concentration of 15 000 mg L-1 upward. Subsequent subculturing was observed to enhance the degradative capabilities of the isolates and the further removal of the higher molecular mass compounds. However, this enhanced degradation was much lower in creosote cultures than in the other cultures.
Similar content being viewed by others
References
Alexander, M.: 1999, Biodegradation and Bioremediation. Academic Press, San Diego. p. 302.
Andrew, J. F.: 1968, ‘A mathematical model for the continuous culture of microorganisms’, Biotech. Bioeng. 10, 707–723.
Arvin, E. and Flyvbjerg, J.: 1992, ‘Groundwater pollution arising from the disposal of creosote waste’, J. IWEM. 6(6), 646–652.
Bartha, R.: 1986, ‘Biotechnology of petroleum pollutant biodegradation’, Microb. Ecol. 12, 155–172.
Bossert, I. D. and Bartha, R.: 1986, ‘Structure-bioderadability relationships of polycylic aromatic hydrocarbons in soil’, Bull. Environ. Contam. Toxicol. 37, 490–495.
Bull, A. T. and Slater, J. H.: 1976, ‘The Teaching of Continuous Culture’, in A. C. R. Dean, C. G. T. Evans and J. Melling (eds). Continuous Culture 6: Application and New Fields, Ellis Hoorwood, Chichester. pp. 49–68.
Cerniglia, C. E. and Heitkamp, M. A.: 1989, ‘Microbial Degradation of PAHs in the Aquatic Environment’, in U. Vuranasi (ed.), Metabolism of PAHs in Aquatic Environment, CRC. Press. Boca Raton, Florida.
Coutts, D. A. P., Senior, E. and Balba, M. T. M.: 1987, ‘Multistage hemostat investigation of interspecies interactions in a hexanoate-catabolizing microbial association isolated from anoxic landfill’, J. Appl. Bact. 62, 251–260.
Ellis, B.: 1994, ‘Reclaiming Contaminated Land: In situ/ex situ Remediation of Creosote-and Petroleum Hydrocarbon-Contaminated Sites’, in P. E. Flatham, D. E. Jerger and J. H. Exner (ed.), Bioremediation: Field experience, Lewis Publishers, London. pp 107–128.
Eriksson, M., Dalhammar, G. and Borg-Karlson, A.-K.: 1999, ‘Aerobic degradation of a hydrocarbon mixture in natural uncontaminated potting soil by indigenous microorganisms at 20 and 6 °C’, Appl. Microbiol. Biotechnol. 51, 532–535.
Gibson, D. T. and Subramanian, V.: 1984, ‘Microbial Degradation of Aromatic Hydrocarbons’, in D. T. Gibson (ed.), Microbial Degradation of Organic Compounds, Marcel Dekker, Inc., New York. p. 182–252.
Jannasch, H. W.: 1967, ‘Enrichment of aquatic bacteria in continuous culture’, Arciv. Für Mikrobiologie. 59, 165–173. JZgenson, B. B.: 1977, ‘Bacterial sulfate-reduction within microniches of oxidized marine sediments’, Mar. Biol. 41, 7-17.
Lajoie, C. A. and Strom, P. F.: 1994, ‘Biodegradation of Polynuclear Aromatic Hydrocarbons in Coal Tar Oil Contaminated Soil’, in D. L. Wise and D. J. Trantolo (eds) Remediation of Hazardous Waste Contaminated Soils, Marcel Dekker Inc. New York.
Lees, Z. M.: 1996, ‘Bioremediation of Oil-Contaminated Soil: A South African Case Study’, PhD Thesis, Department of Microbiology and Plant Pathology, University of Natal, Pietermaritzburg, South Africa. 350 pp.
Mueller, J. G., Chapman, P. J. and Pritchard, P. H.: 1989, ‘Action of a fluoranthene-utilizing bacterial community on polycyclic aromatic hydrocarbon components of creosote’, Appl. Environ. Microbiol. 55, 3085–3090.
Otte, M., Gagnon, J., Comeau, Y., Matte, N., Greer, C. W. and Samson, R.: 1994, ‘Activation of an indigenous microbial consortium for bioaugmentation of pentachlorophenol/creosote contaminated soils’, Appl. Microbiol. Biotechnol. 40, 926–932.
Parkes, R. J.: 1982, ‘Methods for Enriching, Isolating and Analyzing Microbial Communities in Laboratory Systems’, in A. T. Bull and J. H. Slater (eds), Microbial Interactions and Communities. Vol. 1. Academic Press, Jovanovich London. pp. 45–99.
Pirt, S. J.: 1975, Principles of Microbes and Cell Cultivation, Blackwell, Oxford.
Renoux, A. Y., Millette, D., Tyagi, R. D. and Samson, R.: 1999, ‘Detoxification of fluorene, phenanthrene, carbazole and p-cresol in columns of aquifer sand as studied by the Microtox assay’, Water Res. 33(9), 2045–2052.
Trzesicka-Mlynarz, D and Ward, O. P.: 1995, ‘Degradation of polycyclic aromatic hydrocarbons (PAHs) by a mixed culture and its component pure cultures, obtained from PAH-contaminated soil’, Can. J. Microbiol. 41, 470–476.
Veldkamp, H. and Jannasch, H. W.: 1972, ‘Mixed culture studies with the hemostat’, J. Appl. Chem. Biochem. 22, 105–123.
Weissenfels, W. D., Beyer, M. and Klein, J.: 1990, ‘Degradation of phenethrene, fluorene and fluoranthene by pure bacterial cultures’, Appl. Microbiol. Biotechnol. 32, 479–484.
Wodzinski, R. S. and Coyle, J. E.: 1974, ‘Physical state of phenanthrene for utilization by bacteria’, Appl. Environ. Microbiol. 27, 1081–1084.
Wodzinski, R. S. and Bertolini, D.: 1979, ‘Physical state in which naphthalene and bibenzyl are utilized by bacteria’, Appl. Microbiol. 23, 1077–1081.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Atagana, H.I., Haynes, R.J. & Wallis, F.M. Batch Culture Enrichment of Indiginous Soil Microorganisms Capable of Catabolizing Creosote Components. Water, Air, & Soil Pollution 141, 233–246 (2002). https://doi.org/10.1023/A:1021352314118
Issue Date:
DOI: https://doi.org/10.1023/A:1021352314118