Abstract
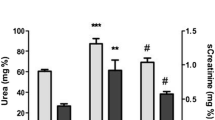
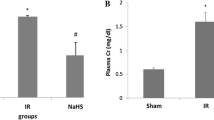
This experimental study was designed to investigate whether midazolam hasantioxidant effects in reperfused rat kidneys following ischemia. TwentyWistar Albino rats were included in the study. Rats were anesthetized withthe mixture of ketamine 90 mg/kg and xylazine 10 mg/kg administeredintraperitoneally. Following anesthesia, the rats were divided into twogroups. The first group was considered as the control group, whereas thesecond group received additional midazolam 3.5 mg/kg intraperitoneally.The left kidney was approached via a transabdominal incision and the leftrenal artery was dissected. Left renal ischemia was created by clamping theleft renal artery for 45 minutes. Following the ischemia period, the kidneywas reperfused for one hour. Both kidneys were then removed. Half of theleft kidneys were immediately immersed in liquid nitrogen fortransportation and then frozen at −70 C until measurements of tissuemalondialdehyde (MDA) and glutathione (GSH) levels. The remaining halvesof the left kidneys and right kidneys were fixed in 10% formalin. Thechanges which developed during the ischemia-reperfusion period in theleft kidney were investigated by histopathological examination andcompared with those of the normal contralateral kidney.When compared with the control group, tissue MDA and GSH levels weresimilar in the midazolam group (p > 0.05). Tubular damage with tubulitis andfocal interstitial inflammatory infiltration were observed inhistopathological examinations of reperfused left kidneys of the controlgroup. There was PMNL infiltration only in perirenal fat tissue of themidazolam group. Right kidneys were histopathologically normal in bothgroups.We concluded that within this dosage midazolam does not have anyantioxidant effect in reperfused rat kidneys following ischemia.
Similar content being viewed by others
References
Bast A, Haenen GR, Doelman CJA. Oxidants and antioxidants: state of art. Am J Med 1991; 91: 2–13.
Thomas MJ. The role of free radicals and antioxidants: how do we know that they are working? Crit Rev Food Sci Nutr 1995; 35(1– 2): 21–39.
Lubec B, Golej J, Marx M et al. L-arginine reduces kidney lipid peroxidation, glycoxidation and collagen accumulation in the aging NMRi mouse. Ren Physiol Biochem 1995; 18(2): 97–102.
Kehrer JP. Free radicals as mediators of tissue injury and disease. Crit Rev Toxicol 1993; 23: 21–48.
Pryor WA. Oxy-radicals and related species. Their formation, lifetimes and reactions. Ann Rev Physiol 1986; 48: 657–667.
Hammond B, Kontos HA, Hess ML. Oxygen radicals in the adult respiratory distress syndrome, in myocardial ischemia and reperfusion injury and in cerebral vascular damage. Can J Physiol Pharmacol 1985; 63: 173–187.
Halliwell B. The role of oxygen radicals in human disease, with particular reference to the vascular system. Heamostasis 1993;23(Suppl 1): 118–126.
Murphy ME, Kehrer JP. Free radicals: a potential pathogenic mechanism in inherited muscular dystrophy. Life Sci 1986; 39: 2271–2278.
Jesberger JA, Richardson JS. Oxygen free radicals and brain dysfunction. Int J Neurosi 1994; 57: 1–17.
Mc Cord JM. Oxygen devired free radicals in postischemic tissue injury. N Engl J Med 1985; 312: 159–163.
Hall E. Lipid antioxidants in acute central nervous system injury. Ann Emerg med 1993; 6: 1022–1027.
Davenport A, Hopton M, Bolton C. Measurement of malondialdehyde as a marker of oxygen free radical production during renal allograft transplantation and the effect on early graft function. Clin Transplant 1995; 9(3 Pt 1): 171–175.
Yaqoob M, McClelland P, Patrick AW et al. Evidence of oxidant injury and tubular damage in early diabetic nephropathy. QJM 1994; 87(10): 601–607.
Naito Y, Yoshikawa T, Matsuyama K et al. Effects of free radical scavengers on indomethacin induced agravation of gastric ulcer in rats. Dig Dis Sci 1995;40(9): 2019–2021.
Bhaumik G, Srivastava KK, Selvamurthy W, Purkayastha SS. The role of free radicals in cold injuries. Int J Biometeorol 1995;38(4): 171–175.
Freeman BA, Crapo JD. Free radicals and tissue injury. Lab Invest 1982; 47: 412–426.
Kaplan P, Racay P, Lehotsky J, Mezesova V. Change in fluidity of brain endoplasmic reticulum membranes by oxygen free radicals: a protective effect of stobadine, alpha tocopherol acetate, and butylated hydroytoluene. Neurochem Res 1995; 20(7): 815–820.
Kahl R. Synthetic antioxidants: biochemical actions and interference with radiation, toxic compounds, chemical mutagens and chemical carcinogens. Toxicology 1984; 33: 185–228.
Haraldsson G, Sorensen V, Nilsson U et al. Effect of pre-treatment with desferioxamine and mannitol on radical production and kidney function after ischemia reperfusion. A study on rabbit kidneys. Acta Physiol Scand 1995; 154(4): 461–468.
Mussacchio E, Rizzoli V, Bianchi M et al. Antioxidant action of propofol on liver microsomes, mitochondria and brain synaptosomes in the rat. Pharmacol and Toxicology 1991; 69: 75–77.
Eriksson O, Pollesello P, Saris NE. Inhibation of lipid peroxidation in isolated rat liver mitochondria by the general anesthetic propofol. Biochem Pharmacol 1992; 44: 391–393.
Murphy PG, Myers DS, Deavies MJ et al. The antioxidant potential of propofol (2.6-diisopropylphenol). Br J Anaesth 1992; 68: 613–618.
Dirnagl U, Lindauer U, Them A et al. Global cerebral ischemia in the rat: online monitoring of oxygen free radical production using chemiluminescence in vitro. J Cereb Flow Metab 1995; 15(6): 929–940.
Flecknell PA. Anaesthesia of animals for biomedical research. Br J Anaesth 1993; 71: 885–894.
Kantos HA, Wei HP. Superoxide production in experimental brain injury. J Neurosurg 1986; 64: 803–807.
Barut S, Canbolat A, Bilge T et al. Lipid peroxidation in experimental spinal cord injury: time-level relationship. Neurosurg Rev 1993; 16: 53–59.
Buege JA, Aust SD. Micosomal lipid peroxidation. Methods Enzymol 1978; 12: 302–310.
Ellman GL. Tissue suphydryl groups. Arch Biochem Biophys 1959; 82: 70–79.
Gurtco HL, Hipkens JH, Sharma SD. Role of glutathione in the metabolism dependent toxicity and chemotherapy of cyclophosphamide. Cancer Research 1981; 41: 3584–3591.
Greene EL, Paller MS. Oxygen free radicals in acute renal failure. Miner Electrolyte Metab 1991; 17(2): 124–132.
Dix TA, Aikens J. Mechanism and biological relevance of lipid peroxidation initiation. Chem Res Toxicol 1993; 6: 2–18.
Smith MI, Thoe H, Orrenius S. The role of lipid peroxidation in the toxicity of foreign compounds to liver cells. Biochem Biopharmacol 1983; 32: 763–764.
Bird BD, Drapper HH. Comperative studies on different methods of malondialdehyde determination. Methods Enzymol 1984; 105: 299–305.
Greene EL, Paller MS. Xanthine oxidase produces O2 – in posthypoxic injury of renal epithelial cells. Am J Physiol 1992; 263(2): 251–255.
Yang CL, Du XH, Han YX. Renal cortical mitochondria are the source of oxygen free radicals enhanced by gentamicin. Ren Fail 1959; 17(1): 21–26.
Ratych RE, Bulkley GB. Free radical mediated postischemic reperfusion injury in the kidney. J Free Radic Biol Med 1986; 2(5– 6): 311–319.
Konya L, Bencsath P, Szenasi G, Feher J. Lack of effect of antixoidant therapy during renal ischemia and reperfusion in dogs. Experientia 1993; 49(3): 253–257.
Li JZ, Wang HY, Tang J, Zou WZ. The effect of calcitonin gene related peptide on acute ischemia reperfusion renal injury: ultrastructural and membrane lipid peroxidation studies. Ren Fail 1992; 14(2): 11–16.
Hall E. Lipid antioxidants in the acute central nervous system injury. Ann Emerg Med 1993; 6: 1022–1027.
Neuromayer HH, Gellert J, Luft FC. Calcium antagonists and renal protection. Ren Fail 1993; 15(3): 353–358.
Paller MS, Eaton JW. Hazards of antioxidant combinations containing superoxide dismutase. Free Radic Biol med 1995; 18(5): 883–890.
Chinev S, Bakalova R, Peneva V et al. Nitrous oxide with fentanyl and droperidol minimizes lipid peroxidation in the liver. Eur J Anaesthesiol 1995; 12(2): 155–162.
Davidson JA, Boom SJ, Pearsall FJ et al. Comparison of the effects of four i.v. anaesthetic agents on polymorphonuclear function. Br J Anaesth 1995; 74(3): 315–318.
Krumholz W, Demel C, Jung S et al. The effects of thiopentone, etomidate, ketamin and midazolam on several bactericidal functions of polymorphonuclear leucocytes in vitro. Eur J Anaesthesiol 1995; 12(2): 141–146.
Weiss M, Buhl R, Medve M, Schneider EM. Tumor necrosis factor alpha modulates the selective interference of hypontics and sedatives to suppress. Crit Care Med 1997; 25(1): 128–134.
Choi SS, Huh KD, Woo JS, Kim YK. Effects of t-butylhydroperoxide on p-aminohippurat uptake in rabbit renal cortical slices. Korean J Intern Med 1994; 9(2): 105–112.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Erol, U., Gurdal, M., Erol, A. et al. Is midazolam effective as an antioxidant in preventing reperfusion injury in rat kidney?. Int Urol Nephrol 34, 121–127 (2002). https://doi.org/10.1023/A:1021338806558
Issue Date:
DOI: https://doi.org/10.1023/A:1021338806558