Abstract
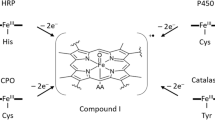
We present a complementary Mössbauer and EPR study on reaction intermediates of substrate-free and substrate-bound cytochrome P450cam from Pseudomonas putida prepared by the freeze-quench method from 57Fe-labeled P450cam using peroxy acetic acid as oxidizing agent. When reacting the substrate-free P450cam for 8 ms reaction time the reaction mixture consists of ∼85% of ferric low-spin iron (Fe(III)) with g-factors and hyperfine parameters of the starting material; the remaining ∼15% are identified as ferryl iron (Fe(IV); S Fe=1) by its Mössbauer signature. Parallel to the ferryl iron a tyrosine radical (S rad=1/2) is formed. The two paramagnetic species are not exchange-coupled; however, they are close enough to significantly influence the (EPR) relaxation behavior of the radical spin. In the case of substrate-bound P450cam only trace amounts of the tyrosine radical are formed within 8 ms (<3%); within the accuracy of Mössbauer spectroscopy (5%) iron(IV) can not be detected. The results point to Tyr-96, which is hydrogen-bonded to the substrate camphor, as the candidate for the observed tyrosine radical.
Similar content being viewed by others
References
Lewis, D. F. V., Cytochromes P450-Structure, Function and Mechanism, Taylor & Francis Ltd, London, 1996.
Schünemann, V., Jung, C., Trautwein, A. X., Mandon, D. and Weiss, R., FEBS Lett. 479 (2000), 149.
Egawa, T., Shimada, H. and Ishimura, Y., Biochem. Biophys. Res. Commun. 201 (1994), 1464.
Wagner, G. C., Palcic, M. M. and Dunford, H. B., FEBS Lett. 156 (1983), 244.
Jung, C., Hui Bon Hoa, G., Schröder, K.-L., Simon, M. and Doucet, J. P., Biochemistry 35 (1992), 12 855.
Wagner, G. C., Perez, M., Toscano Jr., W. A. and Gunsalus, I. C., J. Biol. Chem. 256 (1981), 6262.
Ballou, D. P., Methods Enzymol. 54 (1978), 85.
Beinert, H. and Albracht, S., Biochim. Biophys. Acta 683 (1982), 245.
Trautwein, A. X., Bill, E., Bominaar, E. L. and Winkler, H., Structure and Bonding 78 (1991), 1.
Sharrock, M., Debrunner, P. G., Schulz, C., Lipscomb, J. D., Marshall, V. and Gunsalus, I. C., Biochim. Biophys. Acta 420 (1976), 8.
Poulos, T. L. and Howard, A. J., Biochemistry 26 (1987), 8165.
Poulos, T. L., Finzel, B. C. and Howard, A. J., Biochemistry 25 (1986), 5314.
Loew, G. H. and Harris, D. L., Chem. Rev. 100 (2000), 407.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Schünemann, V., Trautwein, A.X., Jung, C. et al. Mössbauer and EPR Study of Reaction Intermediates of Cytochrome P450. Hyperfine Interactions 141, 279–284 (2002). https://doi.org/10.1023/A:1021255431370
Issue Date:
DOI: https://doi.org/10.1023/A:1021255431370