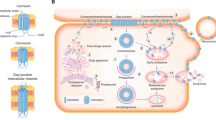
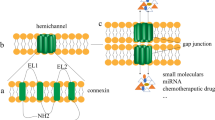
Abstract
Gap junctions, or connexons, are formed by connexin proteins and connect most cells in the body to form water-filled channels directly linking the cytoplasm. Among the molecules known to be transferred via junctions are cAMP, ATP, IP3 and glucose. Tumor cells are in general deficient in functional gap junctions either as a result of gene silencing, or failure to correctly process and assemble connexons. Tumor promoters inhibit function whereas certain cancer preventive agents increase junctional communication. When connexin expression in tumor cells is forced by introduction of exogenous genes or is increased by pharmacological agents, connexin expression reduces growth in suspension and growth as xenografts in nude mice. It is as yet unclear if in tumor cells these actions depend on junctional transfer of signal molecules or reflect some other function of these genes. Restoration of connexin function offers an exciting opportunity to delay tumor progression and inhibit metastasis.
Similar content being viewed by others
References
Beyer EC, Paul DL, Goodenough DA: Connexin family of gap junction proteins. J Membr Biol 116: 187–194, 1990
Kumar NM: Molecular biology of the interactions between connexins. Novartis Found Symp 219: 6–16, 1999
Loewenstein WR, Kanno Y: Intercellular communication and the control of tissue growth: Lack of communication between cancer cells. Nature 209: 1248–1249, 1966
Azarnia R, Loewenstein WR: Intercellular communication and the control of growth: XI. Alteration of junctional permeability by the src gene in a revertant cell with normal cytoskeleton. J Membr Biol 82: 207–212, 1984
Loewenstein WR: Junctional intercellular communication and the control of growth. Biochim Biophys Acta 560: 1–65, 1979
Reznikoff CA, Bertram JS, Brankow DW, Heidelberger C: Quantitative and qualitative studies of chemical transformation of cloned C3H mouse embryo cells sensitive to postconfluence inhibition of cell division. Cancer Res 33: 2339–2349, 1973
Merriman R, Bertram JS: Reversible inhibition by retinoids of 3-methylcholanthrene-induced neoplastic transformation in C3H10T1/2 cells. Cancer Res 39: 1661–1666, 1979
Mordan LJ, Bergin LM, Budnick JL, Meegan R, Bertram JS: Isolation of methylcholanthrene-initiated C3H/10T1/2 cells by inhibiting neoplastic progression with retinyl acetate. Carcinogenesis 3(3): 279–285, 1982
Mordan LJ, Martner JE, Bertram JS: Quantitative neoplastic transformation of C3H/10T fibroblasts: Dependence upon the size of the initiated cell colony at confluence. Cancer Res 43: 4062–4067, 1983
Bertram JS: Modulation of cellular interactions between C3H/10T1/2 cells and their transformed counterparts by phosphodiestrase inhibitors. Cancer Res 39: 3502–3508, 1979
Bertram JS, Faletto MB: Requirements for and kinetics of growth arrest of neoplastic cells by confluent 10T1/2 fibroblasts induced by a specific inhibitor of cyclic adenosine 3:5-phosphodiesterase. Cancer Res 45: 1946–1952, 1985
Mehta PP, Bertram JS, Loewenstein WR: Growth inhibition of transformed cells correlates with their junctional communication with normal cells. Cell 44: 187–196, 1986
Hossain MZ, Wilkens LR, Mehta PP, Loewenstein WR, Bertram JS: Enhancement of gap junctional communication by retinoids correlates with their ability to inhibit neoplastic transformation. Carcinogenesis 10: 1743–1748, 1989
Hossain MZ, Bertram JS: Retinoids suppress proliferation, induce cell spreading, and up-regulate connexin 43 expression only in postconfluent 10T1/2 cells: Implications for the role of gap junctional communication. Cell Growth Differ 5: 1253–1261, 1994
Boutwell RK, Verma AK, Ashendel CL, Astrup E: Mouse skin: A useful model system for studying the mechanism of chemical carcinogenesis. Carcinog Compr Surv 7: 1–12, 1982
Mordan LJ, Bertram JS: Retinoid effects on cell–cell interactions and growth characteristics of normal and carcinogen-treated C3H/10T1/2 cells. Cancer Res 43: 567–571, 1983
Trosko JE, Ruch RJ: Cell–cell communication in carcinogenesis. Frontiers Biosci 3: 208–236, 1998
Ruch RJ, Klaunig JE, Pereira MA: Inhibition of intercellular communication between mouse hepatocytes by tumor promoters. Toxicol Appl Pharmacol [VWO] 87: 111–120, 1987
Moennikes O, Buchmann A, Romualdi A, Ott T, Werringloer J, Willecke K, Schwarz M: Lack of phenobarbital-mediated promotion of hepatocarcinogenesis in connexin 32-null mice. Cancer Res 60: 5087–5091, 2000
Bruzzone R, White TW, Paul DL: Connections with connexins: The molecular basis of direct intercellular signaling. Eur J Biochem 238: 1–27, 1996
Neveu M, Bertram JS: Gap junctions and neoplasia. In: Hertzberg EL, Bittar EE (eds) Gap Junctions, JAI Press, Greenwich, CT, 2000, pp 221–262
Weinberg RA: Tumor suppressor genes. Science 254: 1138–1146, 1991
King TJ, Fukushima LH, Hieber AD, Shimabukuro KA, Sakr WA, Bertram JS: Reduced levels of connexin 43 in cervical dysplasia: Inducible expression in a cervical carcinoma cell line decreases neoplastic potential with implications for tumor progression. Carcinogenesis 21: 1097–1109, 2000
Huang RP, Hossain MZ, Sehgal A, Boynton AL: Reduced connexin 43 expression in high-grade human brain glioma cells. J Surg Oncol 70: 21–24, 1999
Soroceanu L, Manning TJ Jr, Sontheimer H: Reduced expression of connexin-43 and functional gap junction coupling in human gliomas. Glia 33: 107–117, 2001
Aronica E, Gorter JA, Jansen GH, Leenstra S, Yankaya B, Troost D: Expression of connexin 43 and connexin 32 gap-junction proteins in epilepsy-associated brain tumors and in the perilesional epileptic cortex. Acta Neuropathol (Berl) 101: 449–459, 2001
Habermann H, Ray V, Habermann W, Prins GS: Alterations in gap junction protein expression in human benign prostatic hyperplasia and prostate cancer. J Urol 167: 655–660, 2002
Hossain MZ, Jagdale AB, Ao P, LeCiel C, Huang RP, Boynton AL: Impaired expression and posttranslational processing of connexin 43 and downregulation of gap junctional communication in neoplastic human prostate cells. Prostate 38: 55–59, 1999
Robertson KD, Jones PA: DNA methylation: Past, present and future directions. Carcinogenesis 21: 461–467, 2000
Piechocki MP, Burk RD, Ruch RJ: Regulation of connexin 32 and connexin 43 gene expression by DNA methylation in rat liver cells. Carcinogenesis 20: 401–406, 1999
Lee SW, Tomasetto C, Sager R: Positive selection of candidate tumor-suppressor genes by subtractive hybridization. Proc Natl Acad Sci USA 88: 2825–2829, 1991
Tan Lw LW, Bianco T, Dobrovic A: Variable promoter region CpG island methylation of the putative tumor suppressor gene Connexin 26 in breast cancer. Carcinogenesis 23: 231–236, 2002
King TJ, Fukushima LH, Donlon TA, Hieber AD, Shimabukuro KA, Bertram JS: Correlation between growth control, neoplastic potential and endogenous connexin 43 expression in HeLa cell lines: Implications for tumor progression. Carcinogenesis 21: 311–315, 2000
Krutovskikh V, Yamasaki H: Connexin gene mutations in human genetic diseases. Mutat Res 462: 197–207, 2000
Temme A, Buchmann A, Gabriel HD, Nelles E, Schwarz M, Willecke K: High incidence of spontaneous and chemically induced liver tumors in mice deficient for connexin 32. Curr Biol 7: 713–716, 1997
Lampe PD, Lau AF: Regulation of gap junctions by phosphorylation of connexins. Arch Biochem Biophys 384: 205–215, 2000
Saez JC, Martinez AD, Branes MC, Gonzalez HE: Regulation of gap junctions by protein phosphorylation. Braz J Med Biol Res 31: 593–600, 1998
Rivedal E, Opsahl H: Role of PKC and MAP kinase in EGF-and TPA-induced connexin 43 phosphorylation and inhibition of gap junction intercellular communication in rat liver epithelial cells. Carcinogenesis 22: 1543–1550, 2001
Loo LWM, Berestecky JM, Kanemitsu MY, Lau AF: pp60src-mediated phosphorylation of connexin 43, a gap junction protein. J Biol Chem 270: 12751–12761, 1995
Nose A, Tsuji K, Takeichi M: Localization of specificity determining sites in cadherin cell adhesion molecules. Cell 61: 147–155, 1990
Mehta PP, Yamamoto M, Rose B: Transcription of the gene for the gap junctional protein connexin 43 and expression of functional cell-to-cell channels are regulated by cAMP. Mol Biol Cell 3: 839–850, 1992
Gossen M, Freundlieb S, Bender G, Müller G, Hillen W, Bujard H: Transcriptional activation by tetracyclines in mammalian cells. Science 268: 1766–1769, 1995
Mehta PP, Bertram JS, Loewenstein WR: The actions of retinoids on cellular growth correlate with their actions on gap junctional communication. J Cell Biol 108: 1053–1065, 1989
Mehta PP, Hotz Wagenblatt A, Rose B, Shalloway D, Loewenstein WR: Incorporation of the gene for a cell–cell channel protein into transformed cells leads to normalization of growth. J Membr Biol 124: 207–225, 1991
King TJ, Fukushima LH, Yasui, Lampe PD, Bertram JS: Inducible expression of the gap junction protein connexin43 decreases the neoplastic potential of HT-1080 human fibrosarcoma cells in vitro and in vivo. Mol Carcin In press 2003
Giepmans BN, Verlaan I, Hengeveld T, Janssen H, Calafat J, Falk MM, Moolenaar WH: Gap junction protein connexin-43 interacts directly with microtubules. Curr Biol 11: 1364–1368, 2001
Toyofuku T, Yabuki M, Otsu K, Kuzuya T, Hori M, Tada M: Direct association of the gap junction protein connexin-43 with ZO-1 in cardiac myocytes. J Biol Chem 273: 12725–12731, 1998
Xu X, Li WE, Huang GY, Meyer R, Chen T, Luo Y, Thomas MP, Radice GL, Lo CW: Modulation of mouse neural crest cell motility by N-cadherin and connexin 43 gap junctions. J Cell Biol 154: 217–230, 2001
Bertram JS, Bertram BB, Janik P: Inhibition of neoplastic cell growth by quiescent cells is mediated by serum concentration and cAMP phosphodiesterase inhibitors. J Supramol Struct Cell Biochem 18: 515–538, 1982
Janik P, Assaf A, Bertram JS: Inhibition of growth of primary and metastatic Lewis lung carcinoma cells by the phosphodiesterase inhibitor isobutylmethylxanthine. Cancer Res 40: 1950–1054, 1980
Ito A, Katoh F, Kataoka TR, Okada M, Tsubota N, Asada H, Yoshikawa K, Maeda S, Kitamura Y, Yamasaki H, Nojima H: A role for heterologous gap junctions between melanoma and endothelial cells in metastasis. J Clin Invest 105: 1189–1197, 2000
Bechberger JF, Khoo NSK, Naus CCG: Analysis of connexin 43 expression under the control of a metallothionein promoter. Cell Growth Differ 7: 1403–1413, 1996
Singh MV, Bhatnagar R, Malhotra SK: Inhibition of connexin 43 synthesis by antisense RNA in rat glioma cells. Cytobios 91: 103–123, 1997
Mesnil M, Krutovskikh V, Piccoli C, Elfgang C, Traub O, Willecke K, Yamasaki H: Negative growth control of HeLa cells by connexin genes: Connexin species specificity. Cancer Res 55: 629–639, 1995
Goldberg GS, Bechberger JF, Tajima Y, Merritt M, Omori Y, Gawinowicz MA, Narayanan R, Tan Y, Sanai Y, Yamasaki H, Naus CC, Tsuda H, Nicholson BJ: Connexin 43 suppresses MFG-E8 while inducing contact growth inhibition of glioma cells. Cancer Res 60: 6018–6026, 2000
Ek-Vitorín JF, Calero G, Morley GE, Coombs W, Taffet SM, Delmar M: pH regulation of connexin 43: Molecular analysis of the gating particle. Biophys J 71: 1273–1284, 1996
Omori Y, Yamasaki H: Gap junction proteins connexin 32 and connexin 43 partially acquire growth-suppressive function in HeLa cells by deletion of their C-terminal tails. Carcinogenesis 20: 1913–1918, 1999
De Feijter-Rupp HL, Hayashi T, Kalimi GH, Edwards P, Redpath JL, Chang CC, Stanbridge EJ, Trosko JE: Restored gap junctional communication in non-tumorigenic HeLa-normal human fibroblast hybrids. Carcinogenesis 19: 747–754, 1998
Krutovskikh VA, Yamasaki H, Tsuda H, Asamoto M: Inhibition of intrinsic gap-junction intercellular communication and enhancement of tumorigenicity of the rat bladder carcinoma cell line BC31 by a dominant-negative connexin 43 mutant. Mol Carcinog 23: 254–261, 1998
Zhu D, Caveney S, Kidder GM, Naus CC: Transfection of C6 glioma cells with connexin 43 cDNA: Analysis of expression, intercellular coupling, and cell proliferation. Proc Natl Acad Sci USA 88: 1883–1887, 1991
Naus CC, Elisevich K, Zhu D, Belliveau DJ, Del Maestro RF: In vivo growth of C6 glioma cells transfected with connexin 43 cDNA. Cancer Res 52: 4208–4213, 1992
Zhu D, Kidder GM, Caveney S, Naus CC: Growth retardation in glioma cells cocultured with cells overexpressing a gap junction protein. Proc Natl Acad Sci USA 89: 10218–10221, 1992
Bradshaw SL, Naus CCG, Zhu D, Kidder GM, D'Ercole AJ, Han VKM: Alterations in the synthesis of insulin-like growth factor binding proteins and insulin-like growth factors in rat C6 glioma cells transfected with a gap junction connexin 43 cDNA. Regulatory Peptides 48: 99–112, 1993
Ruffin MT, Lancaster WD: Clinical models of chemoprevention for cervical cancer. Hematol Oncol Clin North Am 12: 1115–1134, 1998
Huang RP, Fan Y, Hossain MZ, Peng A, Zeng ZL, Boynton AL: Reversion of the neoplastic phenotype of human glioblastoma cells by connexin 43 (cx43). Cancer Res 58: 5089–5096, 1998
Bond SL, Bechberger JF, Khoo NK, Naus CC: Transfection of C6 glioma cells with connexin 32: The effects of expression of a nonendogenous gap junction protein. Cell Growth Differ 5: 179–186, 1994
Goldberg GS, Lampe PD, Nicholson BJ: Selective transfer of endogenous metabolites through gap junctions composed of different connexins. Nat Cell Biol 1: 457–459, 1999
Zhu D, Kidder GM, Caveney S, Naus CCG: Growth retardation in glioma cells cocultured with cells overexpressing a gap junction protein. Proc Natl Acad Sci USA 89: 10218–10221, 1992
Rose B, Mehta PP, Loewenstein WR: Gap-junction protein gene suppresses tumorigenicity. Carcinogenesis 14: 1073–1075, 1993
Chen SC, Pelletier DB, Ao P, Boynton AL: Connexin 43 reverses the phenotype of transformed cells and alters their expression of cyclin/cyclin-dependent kinases. Cell Growth Differ 6: 681–690, 1995
Yano T, Hernandez-Blazquez FJ, Omori Y, Yamasaki H: Reduction of malignant phenotype of HEPG2 cell is associated with the expression of connexin 26 but not connexin 32. Carcinogenesis 22: 1593–1600, 2001
Yano T, Yamasaki H: Regulation of cellular invasion and matrix metalloproteinase activity in HepG2 cell by connexin 26 transfection. Mol Carcinog 31: 101–109, 2001
Duflot-Dancer A, Mesnil M, Yamasaki H: Dominant-negative abrogation of connexin-mediated cell growth control by mutant connexin genes. Oncogene 15: 2151–2158, 1997
Omori Y, Yamasaki H: Mutated connexin 43 proteins inhibit rat glioma cell growth suppression mediated by wild-type connexin 43 in a dominant-negative manner. Int J Cancer 78: 446–453, 1998
Gabriel HD, Jung D, Butzler C, Temme A, Traub O, Winterhager E, Willecke K: Transplacental uptake of glucose is decreased in embryonic lethal connexin 26-deficient mice. J Cell Biol 140: 1453–1461, 1998
Simpson I, Rose B, Loewenstein WR: Size limit of molecules permeating the junctional membrane channels. Science 195: 294–296, 1977
Bevans CG, Kordel M, Rhee SK, Harris AL: Isoform composition of connexin channels determines selectivity among second messengers and uncharged molecules. J Biol Chem 273: 2808–2816, 1998
Goldberg GS, Lampe PD, Sheedy D, Stewart CC, Nicholson BJ, Naus CC: Direct isolation and analysis of endogenous transjunctional ADP from Cx43 transfected C6 glioma cells. Exp Cell Res 239: 82–92, 1998
Fletcher WH, Byus CV, Walsh DA: Receptor-mediated action without receptor occupancy: A function for cell–cell communication in ovarian follicles. Adv Exp Med Biol 219: 299–323, 1987
Granot I, Dekel N: Cell-to-cell communication in the ovarian follicle: Developmental and hormonal regulation of the expression of connexin 43. Hum Reprod 13(Suppl 4): 85–97, 1998
Simon AM, Goodenough DA, Li E, Paul DL: Female infertility in mice lacking connexin 37. Nature 385: 525–529, 1997
Berridge MJ: The versatility and complexity of calcium signalling. Novartis Found Symp 239: 52–64, 2001
Fry T, Evans JH, Sanderson MJ: Propagation of intercellular calcium waves in C6 glioma cells transfected with connexins 43 or 32. Microsc Res Tech 52: 289–300, 2001
Toyofuku T, Yabuki M, Otsu K, Kuzuya T, Hori M, Tada M: Intercellular calcium signaling via gap junction in connexin-43-transfected cells. J Biol Chem 273: 1519–1528, 1998
Cotrina ML, Lin JHC, Alves-Rodrigues A, Liu S, Li J, Azmi-Ghadimi H, Kang J, Naus CCG, Nedergaard M: Connexins regulate calcium signaling by controlling ATP release. Proc Nat Acad Sci USA 95: 15735–15740, 1998
Jorgensen NR, Geist ST, Civitelli, R, Steinberg TH: ATP-and gap junction-dependent intercellular calcium signaling in osteoblastic cells. J Cell Biol 139: 497–506, 1997
Moon RC, Pritchard JF, Mehta RG, Nomides CT, Thomas CF, Dinger NM: Suppression of rat mammary cancer development by N-(4-hydroxyphenyl)retinamide (4-HPR) following surgical removal of first palpable tumor. Carcinogenesis 10: 1645–1649, 1989
Giepmans BNG, Moolenaar WH: The gap junction protein connexin 43 interacts with the second PDZ domain of the zona occludens-1 protein. Curr Biol 8: 931–934, 1998
Kojima T, Kokai Y, Chiba H, Yamamoto M, Mochizuki Y, Sawada N: Cx32 but not Cx26 is associated with tight junctions in primary cultures of rat hepatocytes. Exp Cell Res 263: 193–201, 2001
Prowse DM, Cadwallader GP, Pitts JD: E-cadherin expression can alter the specificity of gap junction formation. Cell Biol Int 21: 833–843, 1997
Barker RJ, Price RL, Gourdie RG: Increased association of ZO-1 with connexin 43 during remodeling of cardiac gap junctions. Circ Res 90: 317–324, 2002
Yano T, Yamasaki H: Regulation of cellular invasion and matrix metalloproteinase activity in HepG2 cell by connexin 26 transfection. Mol Carcinog 31: 101–109, 2001
Ai ZW, Fischer A, Spray DC, Brown, AMC, Fishman GI: Wnt-1 regulation of connexin 43 in cardiac myocytes. J Clin Invest 105: 161–171, 2000
Bullions LC, Levine AJ: The role of beta-catenin in cell adhesion, signal transduction, and cancer. Curr Opin Oncol 10: 81–87, 1998
Giarre M, Semenov MV, Brown AM: Wnt signaling stabilizes the dual-function protein beta-catenin in diverse cell types. Ann NY Acad Sci 857: 43–55, 1998
Sparks AB, Morin PJ, Vogelstein B, Kinzler KW: Mutational analysis of the APC/beta-catenin/Tcf pathway in colorectal cancer. Cancer Res 58: 1130–1134, 1998
Su L-K, Vogelstein B, Kinzler KW: Association of the APC tumor suppressor protein with catenins. Science 262: 1734–1737, 1993
Papkoff J, Rubinfeld B, Schryver B, Polakis P: Wnt-1 regulates free pools of catenins and stabilizes APC-catenin complexes. Mol Cell Biol 16: 2128–2134, 1996
Sakanaka C, Sun TQ, Williams LT: New steps in the Wnt/beta-catenin signal transduction pathway. Recent Prog Horm Res 55: 225–236, 2000
Meyer RA, Cohen MF, Recalde S, Zakany J, Bell SM, Scott WJ Jr, Lo CW: Developmental regulation and asymmetric expression of the gene encoding Cx43 gap junctions in the mouse limb bud. Develop Genet 21: 290–300, 1997
Makiyama N, Matsui H, Tsuji H, Ichimura K: Attachment and invasion of high-and low-metastatic clones of RCT sarcoma in a three-dimensional culture system. Clin Exp Metastasis 9: 411–425, 1991
Moorby CD: A connexin 43 mutant lacking the carboxyl cytoplasmic domain inhibits both growth and motility of mouse 3T3 fibroblasts. Mol Carcinog 28: 23–30, 2000
Gupta N, Wang H, McLeod TL, Naus CC, Kyurkchiev S, Advani S, Yu J, Perbal B, Weichselbaum RR: Inhibition of glioma cell growth and tumorigenic potential by CCN3 (NOV). Mol Pathol 54: 293–299, 2001
Cesen-Cummings K, Fernstrom MJ, Malkinson AM, Ruch RJ: Frequent reduction of gap junctional intercellular communication and connexin 43 expression in human and mouse lung carcinoma cells. Carcinogenesis 19: 61–67, 1998
Tomai E, Brownell HL, Tufescu TV, Reid K, Campling BG, Raptis L: Gap junctional communication in cultured human lung carcinoma cells. Lung Cancer 23: 223–231, 1999
Jinn Y, Ichioka M, Marumo F: Expression of connexin 32 and connexin 43 gap junction proteins and E-cadherin in human lung cancer. Cancer Lett 127: 161–169, 1998
El Bayoumy K, Rose DP, Papanikolaou N, Leszczynska J, Swamy MV, Rao CV: Cyclooxygenase-2 expression influences the growth of human large and small cell lung carcinoma lines in athymic mice: Impact of an organoselenium compound on growth regulation. Int J Oncol 20: 557–561, 2002
Saito T, Nishimura M, Kudo R, Yamasaki H: Suppressed gap junctional intercellular communication in carcinogenesis of endometrium. Int J Cancer 93: 317–323, 2001
Krutovskikh V, Mazzoleni G, Mironov N, Omori Y, Aguelon A-M, Mesnil M, Berger F, Partensky C, Yamasaki H: Altered homologous and heterologous Gap-junctional intercellular communication in primary human liver tumors associated with aberrant protein localization but not gene mutation of connexin 32. Int J Cancer 56: 87–94, 1994
Yamasaki H, Omori Y, Zaidan-Dagli ML, Mironov N, Mesnil M, Krutovskikh V: Genetic and epigenetic changes of intercellular communication genes during multistage carcinogenesis. Cancer Detect Prev 23: 273–279, 1999
Laird DW, Fistouris P, Batist G, Alpert L, Huynh HT, Carystinos GD, Alaoui-Jamali MA: Deficiency of connexin 43 gap junctions is an independent marker for breast tumors. Cancer Res 59: 4104–4110, 1999
Wilgenbus KK, Kirkpatrick CJ, Knuechel R, Willecke K, Traub O: Expression of Cx26, Cx32 and Cx43 gap junction proteins in normal and neoplastic human tissues. Int J Cancer 51: 522–529, 1992
Lee SW, Tomasetto C, Sager R: Positive selection of candidate tumor-suppressor genes by subtractive hybridization. Proc Natl Acad Sci USA 88: 2825–2829, 1991
Scherer SS, Bone LJ, Deschenes SM, Abel A, Balice-Gordon RJ, Fischbeck KH: The role of the gap junction protein connexin 32 in the pathogenesis of X-linked Charcot-Marie-Tooth disease. Novartis Found Symp 219: 175–185, 1999
Mehta P, Perez-Stable C, Nadji M, Mian M, Asotra K, Roos BA: Suppression of human prostate cancer cell growth by forced expression of connexin genes. Develop Genet 24: 91–110, 1999
Bertram JS: Cellular communication via gap junctions. Sci Med April: 32–41, 2000
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Vine, A.L., Bertram, J.S. Cancer Chemoprevention by Connexins. Cancer Metastasis Rev 21, 199–216 (2002). https://doi.org/10.1023/A:1021250624933
Issue Date:
DOI: https://doi.org/10.1023/A:1021250624933