Abstract
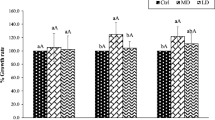
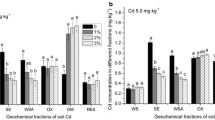
We conducted a pot experiment to investigate the effect of fertilizer additions on the solubility of Cd, Ni and Zn in soil solution and their uptake by plants. Radish (Raphanus sativus cv. Crystal Ball), oat (Avena sativa cv. Thule) and water spinach (Ipomoea aquatica cv. Kangkon) were grown in a naturally metal-rich soil. From day 7 after planting, fertilizers were added daily to each pot. Additions of fertilizer nutrients affected the pH of soil and soil solution, soluble and tissue concentrations of Ca, Mg, K, Cd, Ni and Zn differently in three plant species. The trend of soil and solution pH was in the order: water spinach < radish < oats, that resulted highest soluble and plant tissue concentrations of Cd and Zn in the water spinach followed by radish and then oats. However, Ni concentration in the soil solution increased in all pots and was not affected by pH changes. Soil solution pH increased by more than 1unit in the pots with radish and oats, indicating that mechanisms other than acidification, such as ion exchange and root exudation, may be responsible for the increased heavy metal uptake in these two plant species. Paired t-test showed significantly higher uptake of Cd and Zn in the radish plants resulting in lower concentrations of these elements in the solution. The contribution of mass flow to the supply of major cations and heavy metals varied among elements and plant species. Cadmium, Zn and K were taken up rapidly by all plant species in response to the amount supplied by mass flow. In contrast, the supply of Ni was in excess of its uptake by radish and water spinach. The uptake of all elements was positively correlated (p<0.0001) with mass flow and the transpiration rate in individual plant species. The study suggests that fertilizer cations increased the uptake of metals by improving growth conditions, but the magnitude of increase depended on plant species.
Similar content being viewed by others
References
Barber S.A. 1984. Soil Nutrient Availability. A Mechanistic Approach. John Wiley, New York.
Barber S.A. and Ozanne P.G. 1970. Autoradiographic evidence for the differential effect of four plant species in altering the calcium content of the rhizosphere soil. Soil Sci. Soc. Am. Proc. 34: 635–647.
Brewster J.L. and Tinker P.B. 1970. Nutrient cation flows in soil around plant roots. Soil Sci. Soc. Am. Proc. 34: 421–426.
Brown S.L., Chaney R.I., Angle J.S. and Baker A.J.M. 1994. Zinc and cadmium uptake of Thlaspi caerulescens grown in nutrient solution. Soil Sci. Soc. Am. J. 59: 125–133.
Christensen T. 1984. Cadmium soil sorption at low concentrations: I. Effect of time, cadmium load, pH, and calcium. Water Air and Soil Pollut 21: 105–114.
Eriksson J.E. 1990. Factors influencing Cd levels in soil and in grain of oats and winter wheat: A field study on Swedish soils. In: Swedish Univ., Agric. Sci., Uppsala, Sweden, Report Dissertations. 4..
Elonen P. 1971. Particle size analysis of soil. Acta Agric. Fenn. 122: 1–122.
Finck A. 1982. Fertilizers and Fertilization. Verlag Chemie, Weinheim, West Germany, P. 54, 125.
Hamon R.E., Lorenz S.E., Holm P.E., Christensen T.H. and McGrath S.P. 1995. Changes in trace metals species and other components of the rhizosphere during growth of radish. Plant Cell and Environ. 18: 749–756.
He Q.B. and Singh B.R. 1994. Crop uptake of cadmium from phosphorus fertilizers: I Yield and cadmium content. Water Air and Soil Pollut. 74: 251–265.
Jeng A.S. 1991. Weathering of some Norwegian alum shales: I. Laboratory simulations to study acid generation and the release of sulphate and metal cations (Ca, Mg & K). Acta Agric. Scand. 41: 13–35.
Jeng A.S. and Bergseth H. 1992. Chemical and mineralogical properties of Norwegian alum shale soils, with special emphasis on heavy metal content and availability. Acta Agric. Scand. Sect. B Soil and Plant Sci. 42: 88–93.
Kashem M.A. and Singh B.R. 2001. Metal availability in contaminated soils: I. Effects of flooding and organic matter on the changes in pH, Eh and solubility of Cd, Ni and Zn. Nutr. Cyl. Agro. (in press).
Knight B., Zhao F.J., McGrath S.P. and Shen Z.G. 1997. Zinc and cadmium uptake by the hyperaccumulator Thlaspi caerulescens in contaminated soils and its effects on the concentration and chemical speciation of metals in soil solution. Plant and Soil 197: 71–78.
Lorenz S.E., Hamon R.E., McGrath S.P., Holm P.E. and Christensen T.H. 1994a. Application of fertilizer cations affect cadmium and zinc concentrations in soil solutions and uptake by plants. European J. Soil Sci. 45: 159–165.
Lorenz S.E., Hamon R.E. and McGrath S.P. 1994b. Differences between soil solutions obtained from rhizosphere and non-rhizosphere soils by water displacement and soil centrifugation. European J. Soil Sci. 45: 431–438.
Marschner H. and Römheld V. 1983. In vivo measurement of root-induced pH changes at the soil-root interface: Effect of plant species and nitogen source. Z. Pflanzenphysiol 111: 241–251.
Mengel K. and Krikby E.A. 1987. Principles of plant nutrition. International Potash Institute, Worblaufen-Bern, Switzerland.
Microcal Software Inc. 1997. Origin, Data Analysis and Technical Graphics Release 5. Northampton, MA.
Page A.L., Miller R. and Keeney D.R. 1982. Part 2. Chemical and Microbiological properties. 2nd edn. In: American Society of Agronomy, Inc., Madison, WI, Method of soil Analysis..
Raij B.V. and Diest A.V. 1979. Utilization of phosphate from different sources by six plant species. Plant Soil 51: 577–589.
SigmaPlot, Inc. 1997. SigmaPlot for version 4.00. IPSS.
Singh B.R., Narwal R.P., Jeng A.S. and Almås Å. 1995. Crop uptake and extractability of Cd in soils naturally high in metals at different pH levels. Commun. Soil Sci. Plant Anal. 26: 2123–2142.
Soil Survey Staff 1998. Keys to Soil Taxonomy. 5th edn. U.S. Dep. of Agriculture, Soil Conservations Services.
Strebel O. and Duynisveld W.H.M. 1989. Nitrogen supply in cereals and sugar beet by mass flow and diffusion on a silty loam soil. Pfla. Bodenk. 152: 135–141.
Treeby M., Marschner H. and Romheld V. 1989. Mobilization of iron and other micronutrient cations from a calcareous soil by plant-borne, microbial and synthetic chelators. Plant and Soil 114: 217–226.
Villarroel J.R., Chang A.C. and Amrhein C. 1993. Cd and Zn phytoavailability of a field-stabilized sludge-treated soil. Soil Sci. 155: 197–205.
Willaert G. and Verloo M. 1992. Effects of various nitrogen fertilizers on the chemical and biological activity of major and trace elements in a cadmium contaminated soil. Pedologie 43: 83–91.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kashem, M., Singh, B. The effect of fertilizer additions on the solubility and plant-availability of Cd, Ni and Zn in soil. Nutrient Cycling in Agroecosystems 62, 287–296 (2002). https://doi.org/10.1023/A:1021226201136
Issue Date:
DOI: https://doi.org/10.1023/A:1021226201136