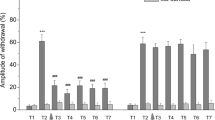
Abstract
Gel shift assays were used to study the formation of transcription factors of the AP-1 family in the CNS of Helix during the acquisition of a conditioned defensive reflex based on food aversion. Increases in the DNA-binding activity of AP-1 factors were seen 1–3 h after training. Modeling of “learning” in an in situ system (incubation of the CNS in the presence of serotonin and the Ca2+ ionophore A23187 or the protein kinase C activator phorbol ester (TPA)) also increased the DNA-binding activity of this transcription complex. The DNA-binding activity induced by serotonin acting alone was significantly less than that in controls, while that in the presence of A23187 and TPA was the same as or less than that in controls. The calcium/calmodulin-dependent protein kinase inhibitor KN62 produced significant suppression of the effects of simultaneous exposure to serotonin and calcium on the activation of transcription factors of the AP-1, while greater suppression was obtained with the mitogen-activated protein kinase (ERK) inhibitor PD98059. Cooperative induction of the activation of AP-1 transcription factors in the CNS of the common snail by the serotonin-induced and calcium-dependent regulatory systems may be a mechanism underlying the formation of conditioned defensive reflexes in these animals. Lesions of the formation of transcription factors of the AP-1 family in animals unable to learn defensive forms of behavioral plasticity may be explained in terms of the presence of inhibitory forms of transcription factors interacting with the SRE element.
Similar content being viewed by others
REFERENCES
K. V. Anokhin, “Molecular scenarios for the consolidation of long-term memory,” Zh. Vyssh. Nerv. Deyat., 47, No. 2, 261–279 (1997).
P. N. Balaban and I. S. Zakharov, Learning and Development - A Common Basis for Two Phenomena [in Russian], Nauka, Moscow (1992).
L. N. Grinkevich, “Protein metabolism in the formation of a defensive reflex in mollusks,” Zh. Vyssh. Nerv. Deyat., 42, No. 6, 1221–1229 (1992).
L. N. Grinkevich, “Formation of C/EBP transcription factors and possible pathways for the regulation of their activity during learning in Helix,” Zh. Vyssh. Nerv. Deyat., 51, No. 1 81–88 (2001).
L. N. Grinkevich and G. V. Vasil'ev, “Possible molecular-cellular mechanisms for the regulation of gene expression in learning,” Ros. Fiziol. Zh., 85, No. 1, 48–66 (1999).
A. Yu. Malyshev, N. I. Bravarenko, A. S. Pivovarov, and P. M. Balaban, “The effects of serotonin levels on postsynaptically induced potentiation of snail neuron responses,” Zh. Vyssh. Nerv. Deyat., 47, No. 3, 553–562 (1997).
V. P. Nikitin, S. A. Kozyrev, and M. O. Samoilov, “Neurophysiological changes and the dynamics of bound calcium during acquisition of associative learning in the common snail,” Neirofiziolog., 24, No. 6, 691–701 (1992).
V. A. Pospelov, T. V. Pospelova, S. B. Tvetlikova, N. A. Kukushkin, and A. van der Éb, “Changes in the composition of transcription factor AP-1 in rat embryo fibroblast cells, transcribed by oncogenes E1A + Ha = Ras,” Mol. Biol., 33, No. 2, 261–267 (1999).
A. V. Shevelkin, V. P. Nikitin, and S. A. Kozyrev, “Serotonin imitates a number of the neuronal effects of nociceptive sensitization in the common snail,” Zh. Vyssh. Nerv. Deyat., 47, No. 3, 432–542 (1997).
C. M. Alberini, M. Ghirardi, R. Metz, and E. R. Kandel, “C/EBP is an immediate-early gene required for the consolidation of long-term facilitation in Aplysia,” Cell, 76, No. 6, 1099–1114 (1994).
P. Angel and M. Karin, “The role of Jun, Fos, and AP-1 complex in cell proliferation and transformation,” Biochem. Biophys Acta, 1072, No. 1, 129–157 (1991).
R. C. Armstrong and M. R. Montminy, “Transsynaptic control of gene expression,” Ann. Rev. Neurosci., 16, No. 1, 17–29 (1993).
D. Bartsch, M. Ghirardi, and P. A. Skehal, “Aplysia CREB 2 represses long-term facilitation and release of repression converts transient facilitation into long-term function and structural change,” Cell, 83, No. 2, 979–992 (1995).
T. Curran and B. R. Franza, “Fos and June: the AP-1 connection,” Cell, 55, 395–397 (1988).
P. K. Dash, B. Hochner, and E. R. Kandel, “Injection of the cAMP-responsive element into the nucleus of Aplysia sensory neurons blocks long-term facilitation,” Nature, 345, 718–721 (1990).
K. Deisseroth, E. K. Heist, and R. W. Tsien, “Translocation of calmodulin to the nucleus supports CREB phosphorylation in hippocampal neurons,” Nature, 392, 198–202 (1998).
I. Demmer, M. Dragunow, and P. A. Lawlor, “Differential expression of immediate early genes after hippocampal long-term potentiation in awake rats,” Mol. Brain Res., 17, No. 3-4, 279–286 (1993).
D. A. Frank and M. E. Greenberg, “CREB: a mediator of long-term memory from molluscs to mammals,” Cell, 79, 5–8 (1994).
L. N. Grinkevich, P. D. Lisachov, I. N. Nagibneva, and L. B. Toporkova, “Intracellular regulation of plasticity in Helix,” Acta Biologica Hungarica, 46, No. 2-4, 263–266 (1995).
R. Janknecht and T. Hunter, “A growing coactivator network,” Nature, 383, 22–24 (1996).
G. Jonat, H. I. Kahmsdorf, K.-K. Park, A. C. B. Cato, S. Gebel, H. Ponta, and P. Herrlich, “Antitumor promotion and antiinflammation. Down-modulation of API (Fos/Jun) activity by glucocortical hormone,” Cell, 62, 1189–1195 (1990).
L. Kaczmarek and A. Chandhuri, “Sensory regulation of immediate early gene expression in mammalian visual cortex: implications for functional mapping and neural plasticity,” Brain Res. Rev., 23, 237–256 (1997).
M. Karin and T. Hunter, “Transcriptional control by protein phosphorylation: signal transmission from the cell surface to the nucleus,” Curr. Biol., 5, 747–757 (1995).
M. Karin, Zh. Liuz, and E. Zandi, “AP-1 function and regulation,” Curr. Opin. Cell Biol., 9, 240–246 (1997).
J. M. Kormhauser and M. E. Greenberg, “A kinase to remember. Dual roles for MAP kinase in long-term memory,” Neuron, 18, No. 6, 839–842 (1997).
K. C. Martin and E. R. Kandel, “Cell adhesion molecules, CREB and the formation of new synaptic connections during development and learning,” Neuron, 17, 567–570 (1996).
A. N. Malviya and P. J. Rogue, “Tell me where is calcium bred. Clarifying the roles of nuclear calcium,” Cell, 92, No. 1, 17–19 (1998).
R. Metzn and E. Ziff, “cAMP stimulates the C/EBP-related transcription factor rNFIL-6 to translocate to the nucleus and induce c-fos transcription,” Genes Dev., 5, 1754–1766 (1991).
M. Montminy and L. M. Bilezikjian, “Binding of a nuclear protein to the cyclic AMP response element of the somatostatin gene,” Nature, 328, No. 6126, 175–178 (1987).
J. I. Morgan and T. Curran, “Stimulus-transcription coupling in the nervous system: involvement of the inducible proto-oncogenes fos and jun,” Ann. Rev. Neurosci., 14, 421–451 (1991).
H. Oshima, D. Srapary, and S. S. Simons, “The factor binding to the glucocorticoid modulatory element of the tyrosine aminotransferase gene is a novel and ubiquitous heteromeric complex,” Biol. Chem., 270, No. 37, 21893–21907 (1995).
V. Sgambato, C. Pages, M. Rogard, M. Besson, and J. Caboche, “Extracellular signal-regulated kinase (ERK) controls immediate early gene induction on corticostriatal stimulation,” J. Neurosci., 18, No. 21, 8814–8825 (1998).
K. L. Thomas, S. Laroche, M. L. Errington, T. V. P. Bliss, and S. P. Hunt, “Spatial and temporal changes in signal transduction pathways during LTP,” Neuron, 13, 737–745 (1994).
J. Xing, D. D. Ginty, and M. E. Greenberg, “Coupling of the RAS-MAPK pathway to gene activation by RSK2, a growth factor-regulated CREB kinase,” Science, 273, 959–963 (1996).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Grinkevich, L.N., Lisachev, P.D. & Merkulova, T.I. Formation of AP-1 Transcription Factors during Learning in Helix. Neurosci Behav Physiol 33, 39–47 (2003). https://doi.org/10.1023/A:1021175230674
Issue Date:
DOI: https://doi.org/10.1023/A:1021175230674